Antimicrobial adhesion surface
a technology of antimicrobial and adhesion surface, which is applied in the direction of synthetic resin layered products, catheters, transportation and packaging, etc., can solve the problems of bacteremia, including septicemia, and the high incidence of nosocomial urinary tract infections of urethral catheterization in hospitalized patients, and achieves the effects of reducing the risk of infection, and improving the safety of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
A first coating composition was prepared by adding the following ingredients successively to a glass beaker under proper agitation until thoroughly mixed.
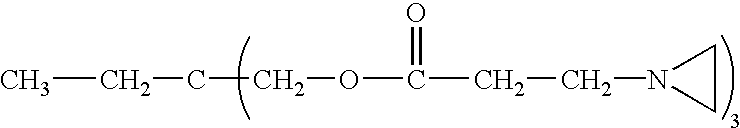
Bayhydrol PR240 (from Bayer) is an aliphatic polyurethane dispersion containing sulfonate groups. The waterborne polyurethane supplied has a pH of 6.5-7.5, and the sulfonate groups are in sodium form. Neocryl CX-100 (from Zeneca resins) is a polyfunctional aziridine crosslinking agent.
A second coating composition, as follows, was prepared as follows:
Glascol (from Allied Colloids) is an acrylic acid / acrylamide copolymer with molecular weight of approximately 6.5 million and pH of 8.0 (1% aqueous solution). The Glascol and sodium chloride were added into the glass beaker with water, and mixed thoroughly under vigorous agitation. After overnight mixing, a clear solution was obtained. Ammonium hydroxide was added into the beaker with an eyedropper to adjust the pH of the solution to 9.0-10.0.
A third coating composition, as follows, was...
example 2
A 6 French urethral stent made of EVA containing 10% Triclosan, an antimicrobial compound (from Ciba-Geigy), was coated with the coating compositions and procedures as described in Example 1. The stent was sterilized by EtO.
example 3
Bacterial adhesion test through dynamic flow model was used to evaluate the coated stents.
The source of infection consisted of a suspension of Escherichia coli at a concentration of 1,000 cfu / mL (or colony forming units per milliliter). Bacterial suspensions were created by inoculating a chemostat with a few colonies of the clinical isolate. The chemostat consisted of a continuously stirred reactor which maintained a steady state bacterial count of 1,000,000 cfu / mL. Trypticase soy broth (BBL, Microbiology Systems, Cockeysville, Md.) was the media used to maintain the bacterial concentration in the chemostat.
The chemostat allows for highly adherent, similar genetric expression, and a consistent daily challenge of cells to be used throughout the course of the experiment.
Daily bacterial counts in the chemostat was confirmed by direct microscopy using acridine orange direct counts. (AODC) Subsequent dilutions in the challenge media of natural urine reflect a consistent 1,000 cfu / mL dail...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hydration time | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
| pulling speed | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com