Compounds and methods for trans-membrane delivery of molecules
A technology of compounds and conjugates, applied in the field of compounds and methods for transmembrane delivery of molecules, capable of solving problems such as heavy charged structures and large OD
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
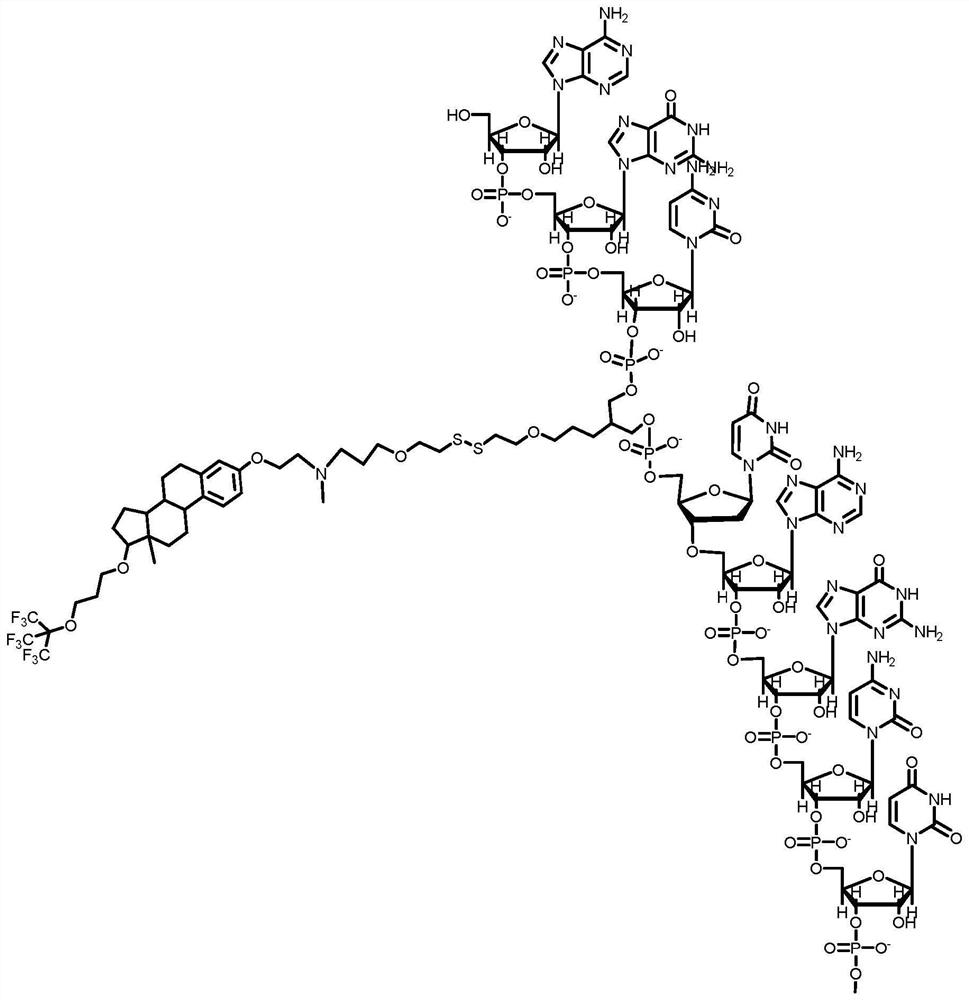
[0326] Example 1: General method for the synthesis of conjugates according to embodiments of the invention, wherein moiety D is an oligonucleoside acid :
[0327] First, genes to be silenced are selected based on their role in disease etiology or pathogenesis. Then, based on bioinformatics methods known in the art, the nucleotide sequence to be incorporated into the conjugate is designed and determined [usually 19-21 base pair double-stranded siRNA for RISC substrates, or for Dicer The substrate (dsiRNA) is a double-stranded RNA of 24-29 base pairs].
[0328] Synthesis proceeds in the 3' to 5' direction of the oligonucleotide. Apply solid phase synthesis using protected building blocks derived from: protected 2'-deoxynucleosides (dA, dC, dG and dT), ribonucleosides (A, C, G and U) or chemical modifications Nucleosides such as [locked nucleic acid (LNA) or bridging nucleic acid (BNA)] . The building blocks are provided as nucleoside precursors in which the 5'- and 3'-h...
example 2
[0330] Example 2: Method for Chemical Synthesis of Precursor Molecules Comprising E, E' or E" Moieties of the Invention :
example 2
[0331] Example 2A: Synthesis of key intermediate phenol 1 :
[0332]
[0333] Treatment of estradiol with excess sodium hydride followed by addition of allyl bromide resulted in complete conversion towards compound 3. Subsequent hydroboration with 1.5 equivalents of 9-BBN yielded only terminal hydroxyl groups, whereas with BH 3 The hydroboration of is much less selective and provides a mixture of adducts. Alcohol 5 was subjected to Mitsunobu reaction conditions to couple it with perfluorinated tert-butanol to give compound 6. Hydrogenolysis of the benzyl group of compound 8 affords phenol 1. In summary, phenol 1 was prepared from estradiol in 45% overall yield via 5 synthetic steps:
[0334] 2bA1.(8R,9S,13S,14S,17S)-3-benzyloxy-17-hydroxyestradiol-1,3,5(10)-triene (2):
[0335] The synthesis of (8R,9S,13S,14S,17S)-3-benzyloxy-17-hydroxyestradiol-1,3,5(10)-triene (2) is disclosed in Section 2aA1 above.
[0336] 2bA2.(8R,9S,13S,14S,17S)-17-allyloxy-3-benzyloxyestra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com