Anti-respiratory syncytial virus fully human broad-spectrum neutralizing antibody 4F1 and application thereof
An antibody and antibody drug technology, applied in the field of medicine, can solve the problems of high price and high dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
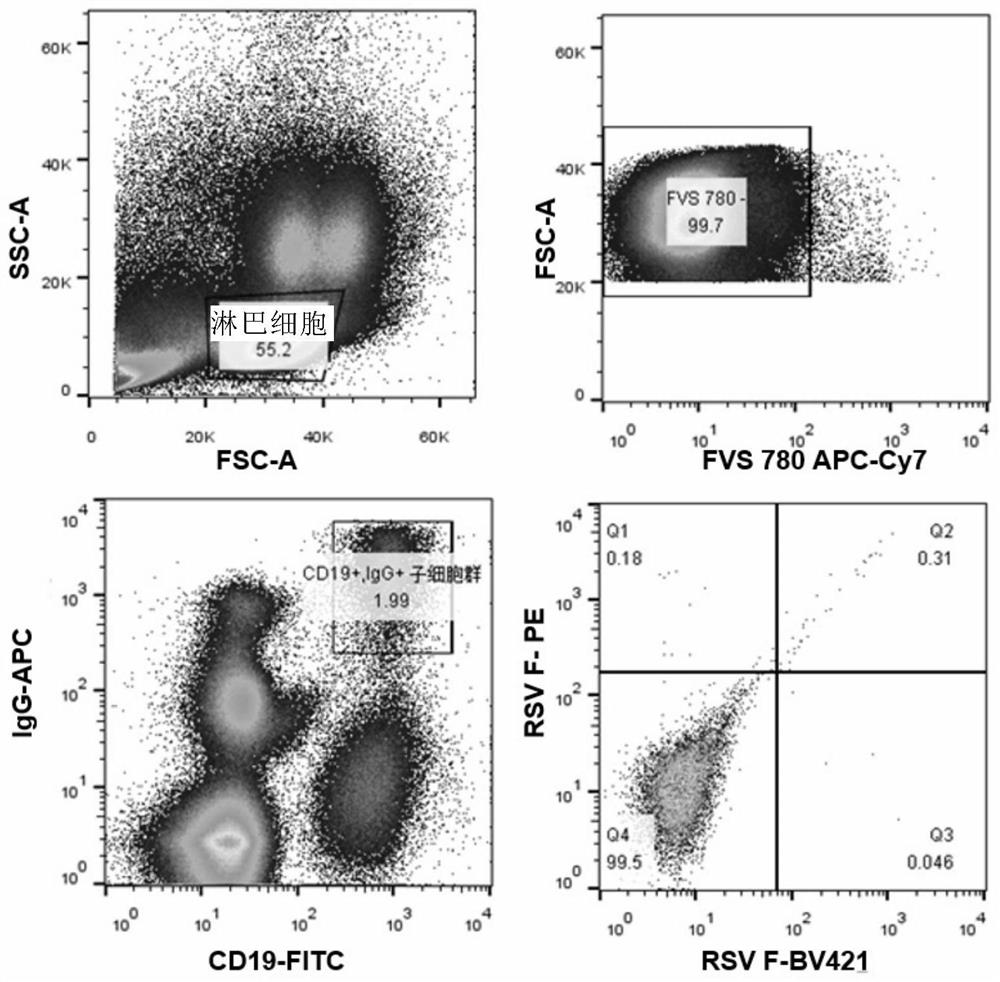
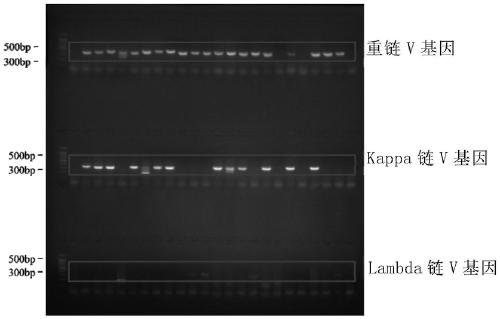
[0193] Example 1 Obtaining antibody gene and antibody expression by single cell RT-PCR method
[0194] 1. Obtaining peripheral blood mononuclear cells (PBMC)
[0195] Peripheral blood was drawn from healthy volunteers, and conventional Ficoll-Paque (manufacturer: -H (CEDARLANE) density gradient centrifugation to obtain 10 7 Above peripheral blood mononuclear cells (PBMC).
[0196] Ficoll separation method:
[0197] (1) Collect blood, collect 20ml of whole blood in a 50ml centrifuge tube (pre-containing 1ml of 4% sodium citrate), invert and mix 8-10 times. (even if the final concentration of sodium citrate is 0.4%);
[0198] (2) Add an equal volume of RPMI1640 (containing sodium citrate), and mix well;
[0199] (3) Use a 15ml transparent centrifuge tube to spread 3ml of lymphocyte separation medium, and carefully add 6ml of blood sample on it. Form a separation interface (or 4ml separation solution plus 8ml blood sample);
[0200] (4) centrifuge at room temperature 800g...
Embodiment 2
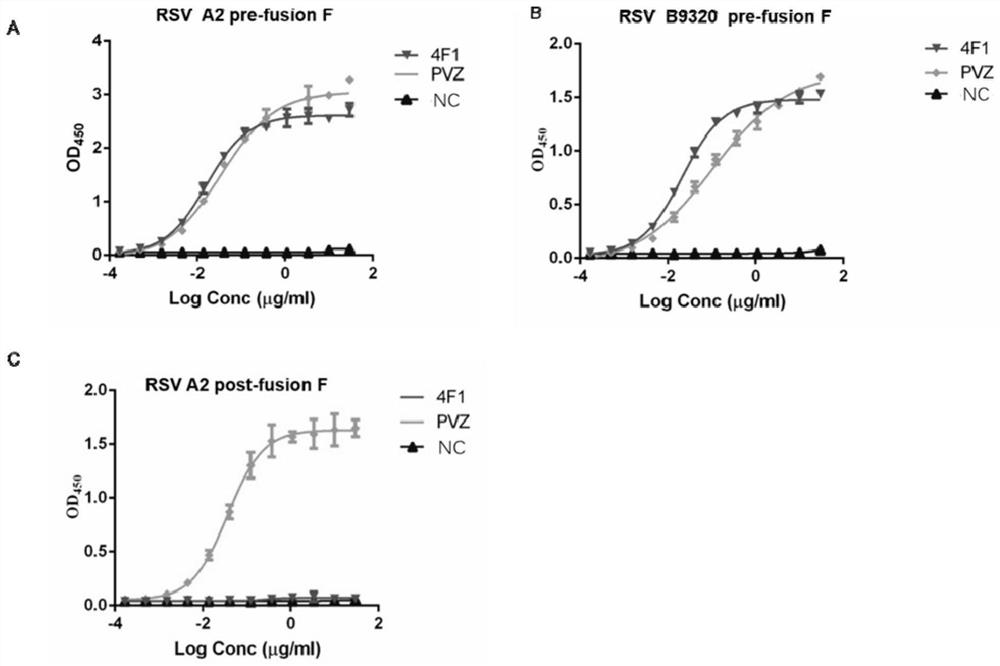
[0226] Example 2 Antibody Characteristic Analysis
[0227] 1. ELISA detection of antibody binding antigen activity
[0228] In order to study the ability of the 4F1 antibody to bind to the F protein of RSV A and B viruses, ELISA was used to detect whether the expressed antibody recognized RSV A2 and B9320 pre-fusion F protein and A2 post-fusion F protein. A2F protein sequence reference UniProtKB / Swiss-Prot:P03420.1, B9320 sequence reference UniProtKB / Swiss-Prot:Q6V2E7, RSV pre-fusion F protein design is based on the strategy adopted by Jason S.McLellan.Science 2013, RSV post-fusion F The protein was designed according to the strategy adopted by Davide Corti.Nature 2013, and the whole gene was synthesized in Shanghai Jierui Company, and constructed on the expression vector of invitrogen pcDNA3.1. Expressed by mammalian cell CHO expression system; refer to Invitrogen ExpiCHO-S TM Expression System Handbook. Palivizumab (Palivizumab, ) is a positive control antibody, purchas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com