Whole-humanized neutralizing antibody resisting H3N2 influenza virus and application of whole-human neutralizing antibody
A technology of antibodies and antibody drugs, which is applied in the field of medicine and can solve problems such as limited applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0188] Example 1 Obtaining antibody gene and antibody expression by single cell RT-PCR method
[0189] 1. Obtaining peripheral blood mononuclear cells (PBMC)
[0190] Peripheral blood was drawn from volunteers screened according to the hemagglutination inhibition test, and conventional Ficoll-Paque density gradient centrifugation was used to obtain 10 7 Above peripheral blood mononuclear cells (PBMC).
[0191] 2. HA-specific memory B cell sorting
[0192] Using FITC-CD19 / APC-IgG / Cy3-HA as a marker, specific B cells were obtained by flow cytometry to a 96-well RT-PCR plate, one cell per well, and several HA-specific memory B cells were obtained.
[0194] Use RT-PCR and Nested-PCR techniques to obtain the antibody gene (heavy and light chain variable region gene), connect the variable region of the antibody heavy and light chain gene from the same B cell to the T vector, and perform sequencing to verify the antibody gene. Then the heavy and light ch...
Embodiment 2
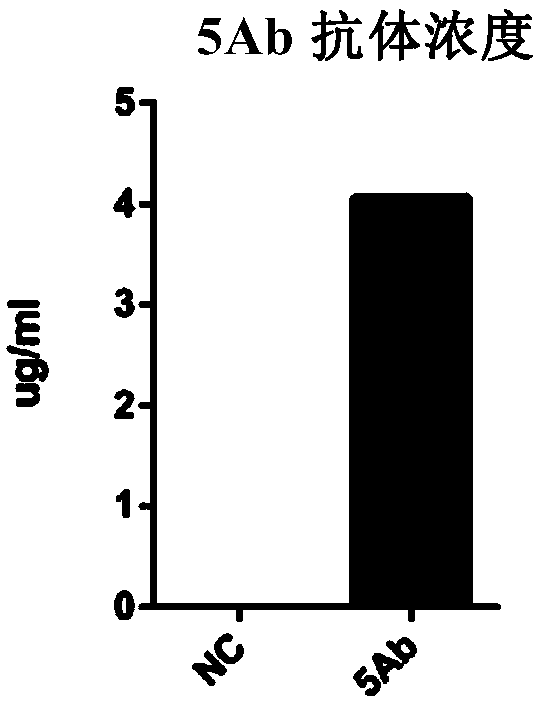
[0225] Example 2 Antibody Characteristic Analysis
[0226] 1. Antigen Specific Detection
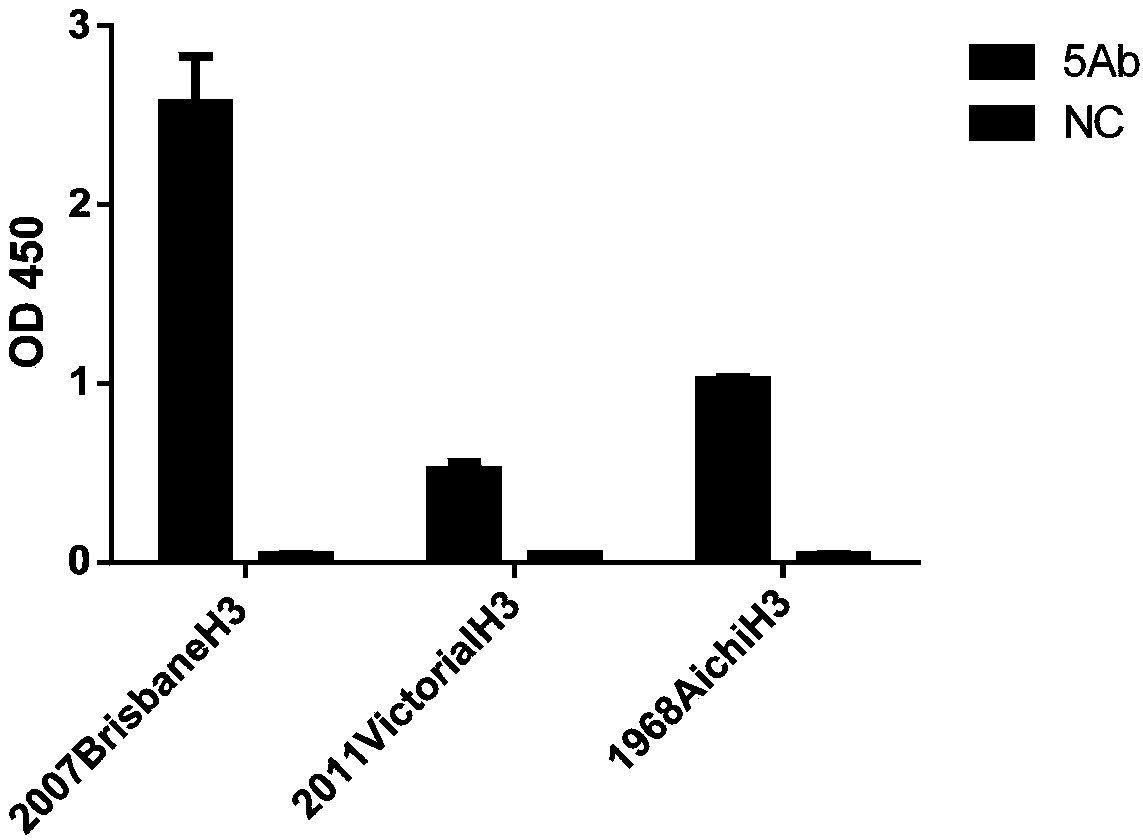
[0227] Detect whether the expressed fully human antibody recognizes HA protein and H3N2 influenza virus vaccine lysate. For detection by ELISA, coat HA and H3N2 influenza vaccine lysates on ELISA plates respectively, 10 μg / ml, 100 μl per well, overnight at 4°C;
[0228] 1) Wash the plate with PBST, 3 times;
[0229] 2) Blocking: 1% BSA, 200 μl per well, 37°C, 2h;
[0230] 3) Wash the plate with PBST, 3 times;
[0231] 4) According to the measured concentration, adjust the whole human antibody cells to the same concentration, 100 μl per well, 37°C, 2h;
[0232] 5) Wash the plate with PBST, 3 times;
[0233] 6) Goat anti-human IgG (Fc specific)-Peroxidase antibody, diluted 1:10000, 100 μl per well was added to the ELISA plate, 37°C, 1h;
[0234] 7) Wash the plate with PBST, 3 times;
[0235] 8) Substrate A solution: B solution = 1:1, 100 μl per well, 37 ° C, 15 min, dark reaction; ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com