Anti-respiratory syncytial virus fully human broad-spectrum neutralizing antibody 4f1 and its application
A syncytial virus, anti-respiratory technology, applied in the field of medicine, can solve the problems of high price and high dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
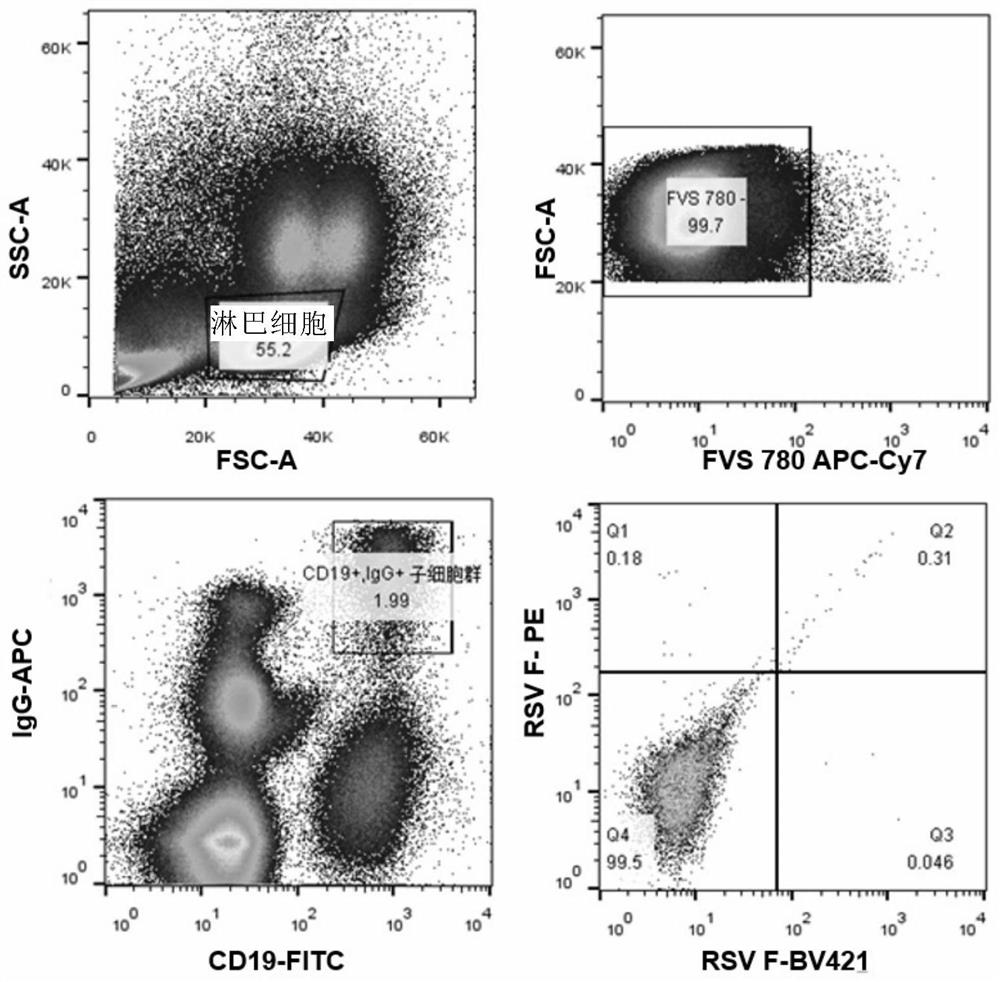
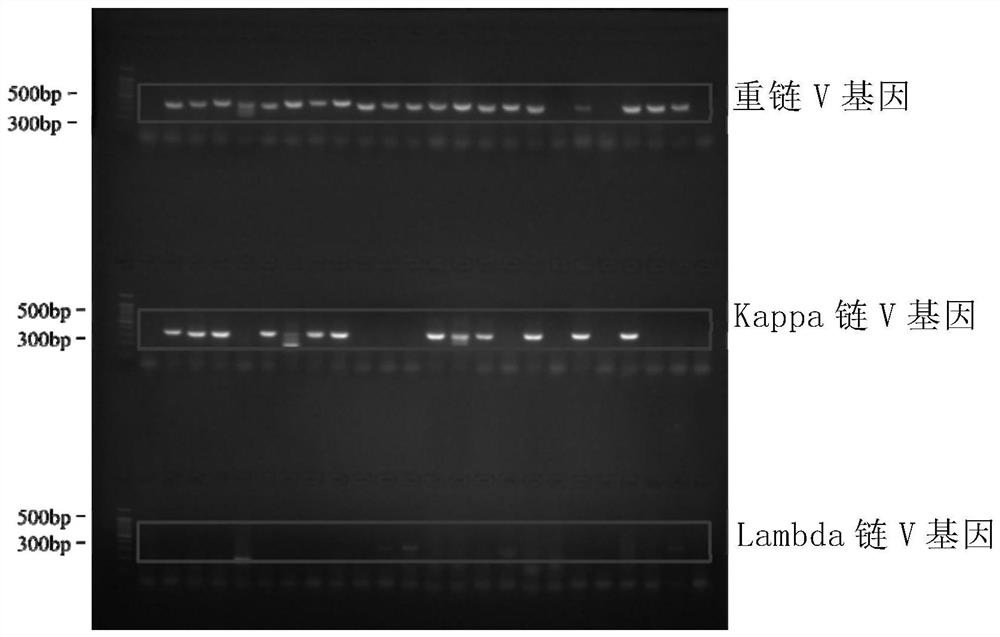
[0193] Example 1 Obtaining antibody genes and antibody expression by single-cell RT-PCR method
[0194] 1. Acquisition of peripheral blood mononuclear cells (PBMC)
[0195] Peripheral blood was drawn from healthy volunteers, using conventional Ficoll-Paque (manufactured by -H (CEDARLANE) density gradient centrifugation to obtain 10 7 The above peripheral blood mononuclear cells (PBMC).
[0196] Ficoll separation method:
[0197] (1) Collect blood, collect 20ml of whole blood in a 50ml centrifuge tube (pre-containing 1ml of 4% sodium citrate), invert and mix 8-10 times. (even if the final concentration of sodium citrate is 0.4%);
[0198] (2) add an equal volume of RPMI1640 (containing sodium citrate), and mix;
[0199] (3) Using a 15ml transparent centrifuge tube, spread 3ml of lymphocyte separation solution, and carefully add 6ml of blood sample on it. Form a separation interface (or 4ml separation solution plus 8ml blood sample);
[0200] (4) Centrifuge 800g at room ...
Embodiment 2
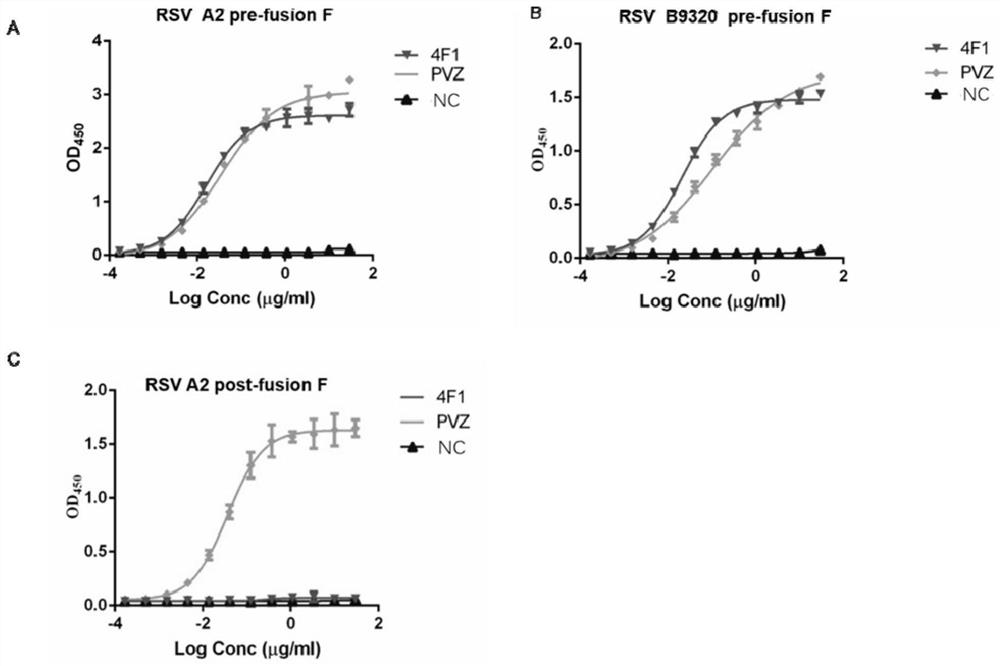
[0226] Example 2 Antibody Characterization Analysis
[0227] 1. ELISA detects the activity of antibody binding to antigen
[0228] To investigate the ability of 4F1 antibody to bind to RSV type A and B virus F proteins, ELISA was used to detect whether the expressed antibodies recognized RSV A2 and B9320 pre-fusion F proteins and A2 post-fusion F proteins. The A2F protein sequence is referred to UniProtKB / Swiss-Prot: P03420.1, and the B9320 sequence is referred to UniProtKB / Swiss-Prot: Q6V2E7. The design of RSV pre-fusion F protein is based on the strategy adopted by Jason S. McLellan. Science 2013. RSV post-fusion F The protein was designed according to the strategy adopted in Davide Corti.Nature 2013. The whole gene was synthesized in Shanghai Jierui Company and constructed into the expression vector of invitrogen pcDNA3.1. Expressed by mammalian cell CHO expression system; refer to Invitrogen ExpiCHO-S TM Expression System Handbook. Palivizumab (Palivizumab, ) is the p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com