Anti-HIV polypeptide modified by high molecular weight PEG and preparation method and application thereof
A molecular weight and peptide chain technology, applied in the field of anti-HIV peptides and its preparation, can solve the problems of poor water solubility, less research on macromolecular modification, and short half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0150] Embodiment 1: PEGylated polypeptide PEG 10k Preparation of NC
[0151] At room temperature, 10mg (0.005mmol) mPEG 10k The mixture of Mal and 10 mg (0.0025 mmol) of polypeptide C34NC was dissolved in 10 mL of sodium dihydrogen phosphate / disodium hydrogen phosphate buffer solution (50 mM, pH 8.0-9.0), and the reaction was monitored by HPLC until the reaction of polypeptide C34NC was complete.
[0152] The obtained PEG-modified polypeptide was purified by Agilent 1200 reversed-phase high-performance liquid chromatography. Chromatographic column model: Angilent Eclipse XDB-C8 Semi-Prep, 5μm, 9.4×250mm; eluent composition: mobile phase A (aqueous solution containing 0.1% trifluoroacetic acid by volume), mobile phase B (including volume The percentage concentration is 0.1% trifluoroacetic acid in acetonitrile solution); elution conditions: A linear gradient elution from 40% to 70%, time 11min, elution flow rate 2mL / min, ultraviolet detection wavelength 220nm. Collect the p...
Embodiment 2
[0156] Example 2: PEGylated Polypeptide PEG 20k Preparation of NC
[0157] Except mPEG in embodiment 1 10k Mal to mPEG 20k Besides Mal, prepare, purify and characterize according to the method in embodiment 1, obtain PEG 20k NC (purity is 97.5%), mass spectrometry results are as figure 2 shown.
[0158] Obtained PEG 20k The structure of NC is:
[0159]
Embodiment 3
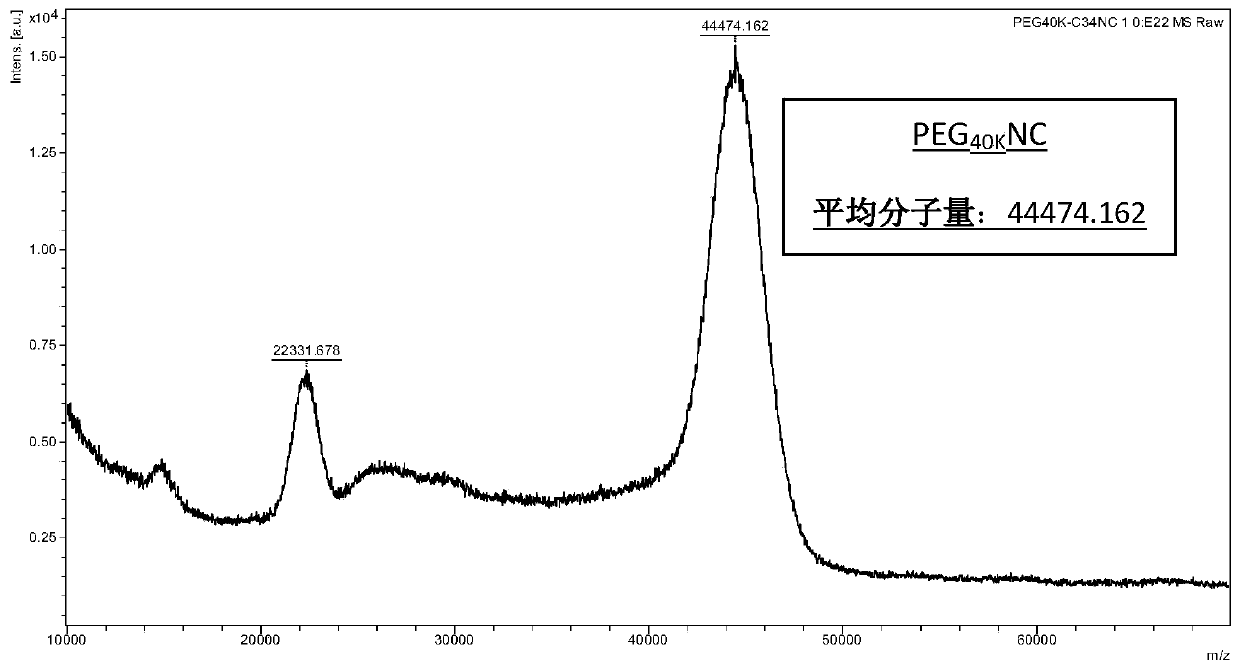
[0160] Example 3: PEGylated Polypeptide PEG 40k Preparation of NC
[0161] Except mPEG in embodiment 1 10k Mal to mPEG 40k Besides Mal, prepare, purify and characterize according to the method in embodiment 1, obtain PEG 40k NC (purity is 98%), mass spectrometry results are as image 3 shown.
[0162] Obtained PEG 40k The structure of NC is:
[0163]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com