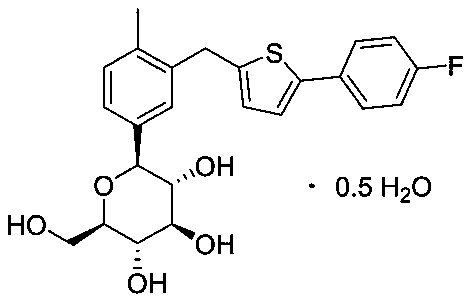
Preparation method of high-purity canagliflozin intermediate
An intermediate and high-purity technology, applied in the field of medicinal chemistry, can solve the problems of low purity and achieve high purity, easy operation and good yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1, a kind of preparation method of high-purity canagliflozin intermediate, described preparation method comprises the following steps:
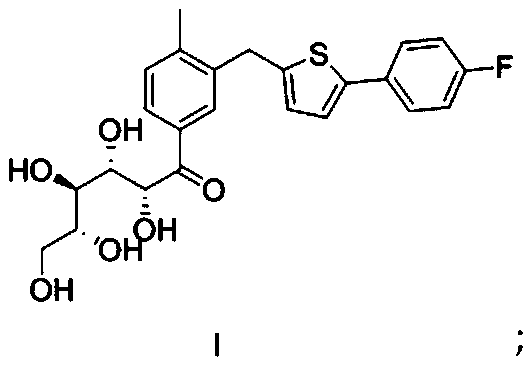
[0046] (1) Under an inert environment, dissolve the thiophene compound in the organic solvent A, add an alkaline reagent under low temperature conditions, and add 2,3,4,6-tetra-O-(trimethylsilyl)-D after the reaction is complete -Condensation reaction of the mixed solution of gluconolactone and organic solvent B; then drop strong acid and water, add alkaline solution after the reaction to quench, separate the organic phase, concentrate, add organic solvent C to crystallize, and dry to obtain the compound of formula I That is, intermediate I;
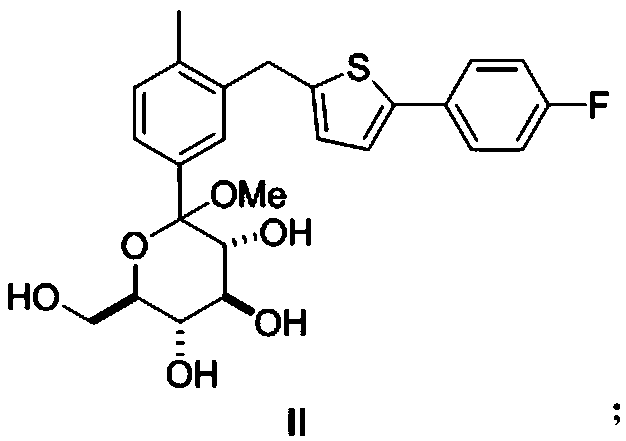
[0047] (2) After intermediate I reacts with strong acid in methanol, add alkaline solution to quench, then add organic solvent D for extraction, after liquid separation, the organic phase is concentrated, add organic solvent E to crystallize to obtain formula II canagliflozin intermedi...
Embodiment 2
[0060] Embodiment 2, a kind of preparation method of high-purity canagliflozin intermediate, described preparation method comprises the following steps:
[0061] (1) Under an inert environment, dissolve the thiophene compound in the organic solvent A, add an alkaline reagent under low temperature conditions, and add 2,3,4,6-tetra-O-(trimethylsilyl)-D after the reaction is complete -Condensation reaction of the mixed solution of gluconolactone and organic solvent B; then drop strong acid and water, add alkaline solution after the reaction to quench, separate the organic phase, concentrate, add organic solvent C to crystallize, and dry to obtain the compound of formula I That is, intermediate I;
[0062] (2) After intermediate I reacts with strong acid in methanol, add alkaline solution to quench, then add organic solvent D for extraction, after liquid separation, the organic phase is concentrated, add organic solvent E to crystallize to obtain formula II canagliflozin intermedi...
Embodiment 3
[0073] Embodiment 3, the preparation method experiment 1 of the high-purity canagliflozin intermediate:
[0074] 1. (2R,3S,4R,5R)-1-{3-[(5-(4-fluorophenyl)thiophen-2-yl)methyl]-4-methylphenyl}-2,3, The preparation of 4,5,6-pentahydroxyhexan-1-one (formula I):
[0075] 20.0g 2-(4-fluorophenyl)-5-[(5-bromo-2-methylphenyl)methyl]thiophene, 200mL tetrahydrofuran and 60mL toluene were added to the reaction flask, stirred to dissolve, nitrogen protection, in At -80~-70°C, add 22mL of n-butyllithium (2.5M n-hexane solution) dropwise, and react for 1h; maintain the temperature, add 28.5g of 2,3,4,6-tetra-O-(trimethyl Silylyl)-D-gluconolactone / 50mL toluene mixture, react for 1h after dropping; add 9.5g trifluoroacetic acid / 20mL water mixture, add and rise to room temperature for reaction. After the reaction is completed, add saturated aqueous sodium bicarbonate solution to adjust the pH to 6-8, separate the two phases after standing for stratification, and add water to the organic ph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com