Preparation method of injectable polyethylene glycol hydrogel loaded with alendronate sodium and application thereof
A technology of sodium alendronate and polyethylene glycol, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, and can solve problems such as side effects, large dosage, and increased dosage , to achieve the effect of maintaining bone structure, improving mineralization process, and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
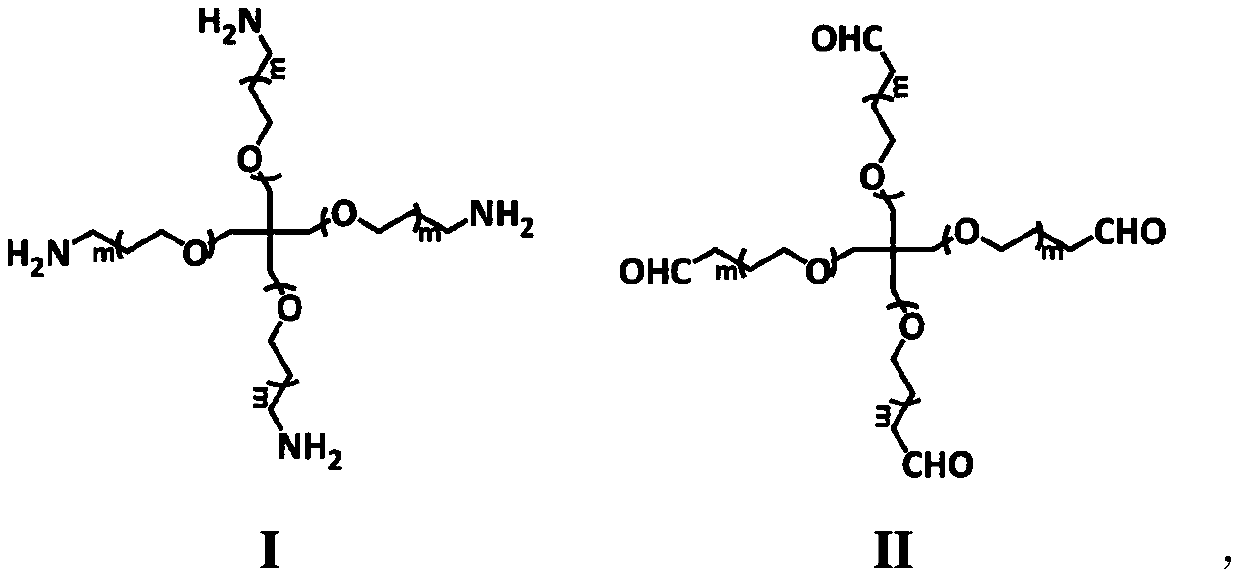
[0041] Weigh 300 mg of four-arm-polyethylene glycol-amino and 0.1 mg of alendronate sodium, mix and dissolve in 1 mL of pure water, then weigh 350 mg of four-arm-polyethylene glycol-aldehyde and dissolve in another 1 mL of pure in water, such as figure 1 As shown, draw a mixed solution with one of the double-barrel syringes, inject the gel into the sample bottle with the double-way syringe, then invert the sample bottle, and record the time. The time when the gel will not flow back is the gelation time. .
[0042] Two gels (four-armed polyethylene glycol hydrogel and four-armed polyethylene glycol hydrogel loaded with alendronate sodium) were subjected to hydrogel toxicity assay:
[0043] (1) The cytotoxicity of hydrogel was determined by CCK-8 kit.
[0044] (2) Prepare rabbit P3-PB-MSCs suspension, inoculate in 96-well plate at a density of 2×103 cells / 100 μL / well, and culture in an incubator at 37°C and 5% CO2 for 12 hours to obtain a monolayer paste parietal cells.
[0...
Embodiment 2
[0049] Weigh 800mg of four-arm-polyethylene glycol-amino and 1mg of alendronate sodium, mix and dissolve in 2mL of pure water, then weigh 1000mg of four-arm-polyethylene glycol-aldehyde and dissolve in another 2mL of pure water In the middle, use one of the double-barreled syringes to draw a mixed solution, and use the double-barrelled syringe to inject it into the bilateral femurs of the osteoporosis rabbits (that is, the drug-loaded gel group), and detect the bone density of the rabbits after 3 months (QCT). The results showed that the bone mineral density of the femoral head in the drug-loaded gel group was significantly higher than that in the blank control group (normal saline), the blank gel group (without alendronate sodium) and the systemic administration group (without gel-forming components) . The results are shown in Table 1.
[0050] Table 1. Comparison table of QCT bone mineral density in each group after treatment
[0051]
Embodiment 3
[0053] Weigh 2000mg of four-arm-polyethylene glycol-amino and 5mg of alendronate sodium, mix and dissolve in 4mL of pure water, then weigh 2400mg of four-arm-polyethylene glycol-aldehyde and dissolve in another 4mL of pure water In the study, one of the double-barrel syringes was used to draw a mixed solution, and the double-barrel syringe was used to inject it into the left femur of the osteoporotic sheep (that is, the drug-loaded gel group), and the bone density of the sheep was detected after 3 months. (DEXA). The results showed that the bone mineral density of the left femoral head of the drug-loaded gel group was higher than that of the blank control group (normal saline), the blank gel group (without alendronate sodium) and the systemic administration group (without gel-forming components). obviously increase.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com