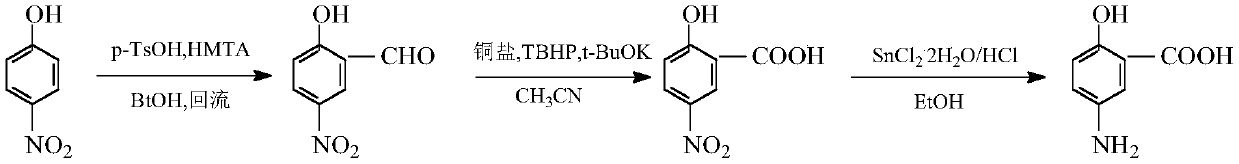
Method for synthesizing mesalazine
A technology of quality and nitrosalicylaldehyde, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve problems such as pollution and high cost, and achieve simple operation and post-processing, short reaction time and low cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
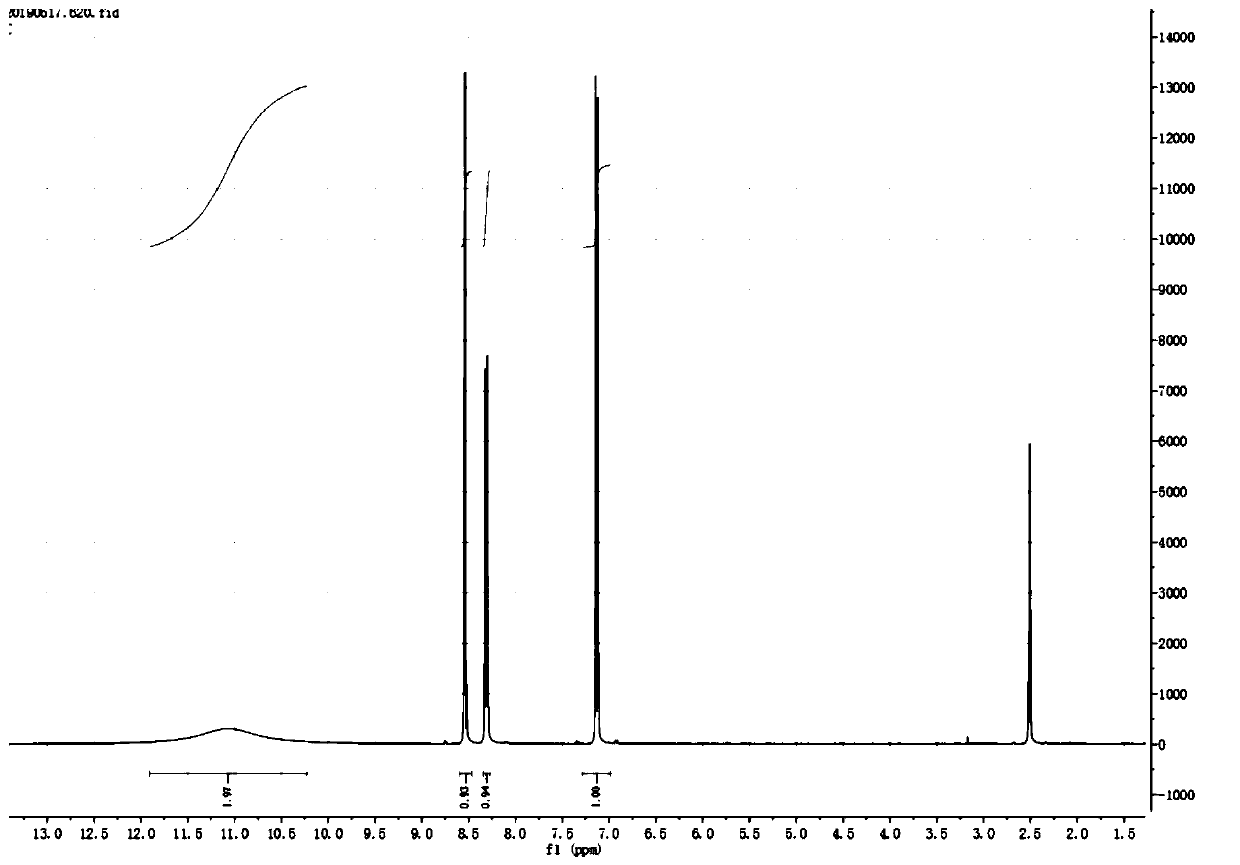
[0038] Add p-nitrophenol (14g, 0.1mol), p-toluenesulfonic acid (26g, 0.15mol) and absolute ethanol (150ml) in sequence in a four-necked reaction flask, slowly raise the temperature to 65°C, and add hexamethylene in 3 batches Tetramine (21g, 0.15mol) was reacted at 78°C for 2h. After the reaction was completed, the heating was stopped, and ice water was poured into the reaction solution, and it was raised to room temperature under stirring. A light yellow solid was precipitated, filtered, washed, and dried to obtain Pale yellow solid 5-nitrosalicylaldehyde (15.5g, 86.5%), purity 96.91%, 1 HNMR schematic diagram as figure 1 shown.
Embodiment 2
[0040] Add p-nitrophenol (14g, 0.1mol), p-toluenesulfonic acid (34.7g, 0.2mol) and absolute ethanol (150ml) in sequence in a four-necked reaction flask, slowly raise the temperature to 78°C, and add hexaethylene in 3 batches Methyltetramine (56g, 0.4mol), reacted at 78°C for 2h. After the reaction was completed, stop heating, pour ice water into the reaction solution, and raise it to room temperature under stirring. A light yellow solid precipitated, filtered, washed, and dried. 5-nitrosalicylaldehyde (16.7 g, 89.4%) was obtained as a pale yellow solid with a purity of 95.42%.
[0041] (2) Preparation of 5-nitrosalicylic acid
Embodiment 3
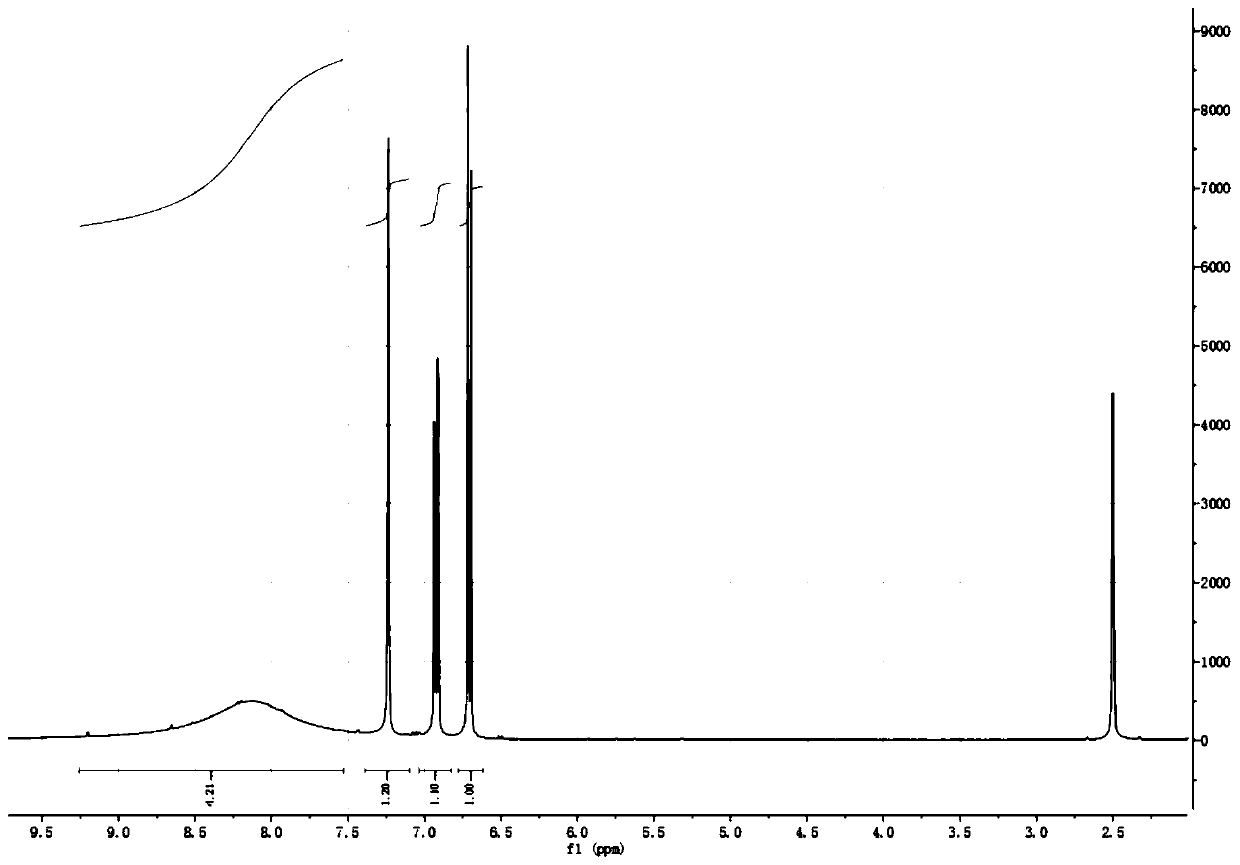
[0043] Add 5-nitrosalicylaldehyde (16.7g, 0.1mol), potassium tert-butoxide (22.4g, 0.2mol), copper bromide (1.1g, 5mmol) and acetonitrile (250ml) successively in the reaction flask, stir Add 70% tert-butyl hydroperoxide (26g, 0.1mol) dropwise and react at 80°C for 5h. After the reaction is over, concentrate under reduced pressure to remove the solvent, pour cold water into the residue, stir, and filter with suction. The filtrate is washed with hydrochloric acid Adjust the pH to 2-3, filter with suction, and dry to obtain 5-nitrosalicylic acid (13.6 g, 89.34%) as a pale yellow solid with a purity of 97.31%. 1 HNMR schematic diagram as figure 2 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com