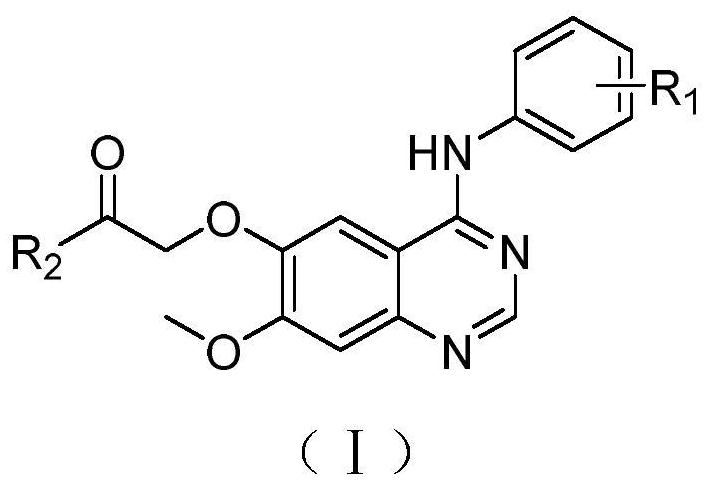
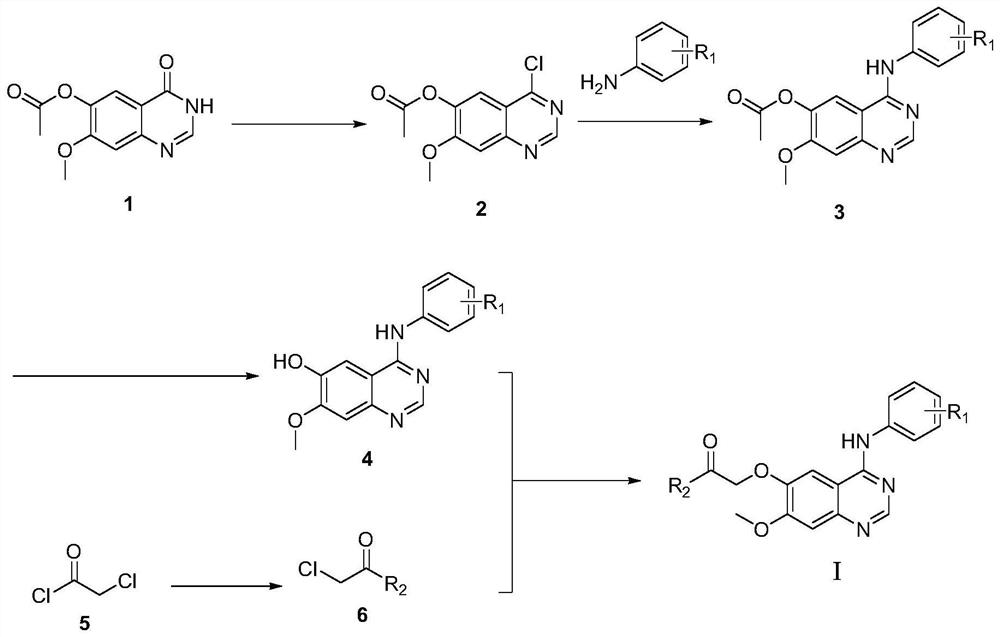
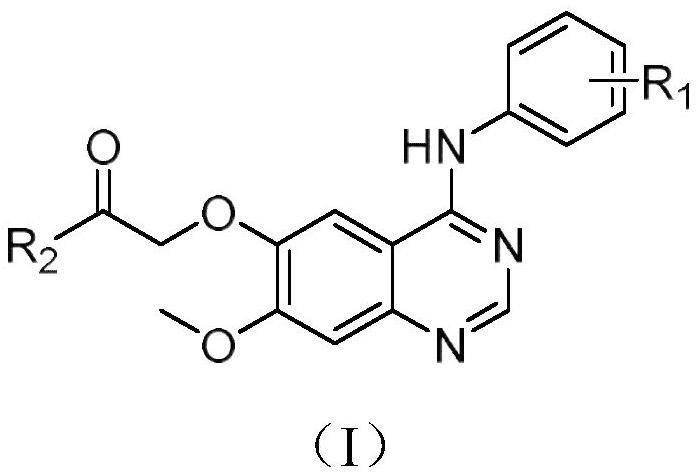
Quinazoline derivatives and their application as antitumor drugs
A quinazoline and derivative technology, applied in the field of drug synthesis, can solve the problems of drug resistance, poor clinical efficacy and the like, and achieve the effects of good inhibitory activity, good selectivity and good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Synthesis of 2-(7-methoxy-4-anilino)quinazolin-6-yl)-1-morpholin-1-one (I-a);
[0044]
[0045] With toluene (30mL) as solvent, 3,4-dihydro-7-methoxy-4-oxoquinazolin-6-ol acetate 1 (3.0g, 12.81mmol), triethylamine (3.1 mL, 21.78mmol) and phosphorus oxychloride (3mL, 32.02mmol) were sequentially added into a 150mL single-necked bottle, and reacted for about 3 hours under constant temperature stirring and reflux at 78°C. The progress of the reaction was monitored by TLC (ethyl acetate / petroleum ether=1 / 1). After the raw materials are completely reacted, the suspension in the bottle is not processed, and the second-step reaction is carried out directly. A solution of aniline a (1.55 g, 16.67 mmol) diluted with toluene (40 mL) was slowly dropped into the above suspension, and stirring was continued for 4 h, resulting in a large amount of solids. TLC monitoring (ethyl acetate / petroleum ether=2 / 1). After the reaction was completed, the reaction liquid was lowered to roo...
Embodiment 2
[0050] Synthesis of 2-(4-(2-fluorophenyl)amino)-7-methoxyquinazolin-6-yl)-1-morpholin-1-one (I-b);
[0051]
[0052] Prepared according to the same method as in Example 1, replacing the raw material aniline with o-fluoroaniline, and synthesized 0.109 g of the target compound (I-b) through 5 steps of chlorination, amination, ester hydrolysis, amidation, and alkylation, with a yield of 73 %; m.p.: 105.7~106.6℃; IR(v,cm -1 ):3293.84, 2918.66, 2854.35, 1640.20, 1504.79, 1424.69, 1206.89, 998.80, 844.15; 1 H-NMR(600MHz,DMSO-d6):δ9.38(s,1H,NH),8.36(s,1H,C=NH),7.78(s,1H,ArH),7.57(s,1H,ArH) ,7.32(d,J=7.3Hz,2H,ArH),7.26(s,1H,ArH),7.22(s,1H,ArH),4.96(s,2H,OCH 2 C=O),3.95(s,3H,OCH 3 ),3.66(s,2H,OCH 2 ),3.60(s,2H,OCH 2 ),3.53(s,2H,NCH 2 ),3.50(s,2H,NCH 2 ).
Embodiment 3
[0054] Synthesis of 2-(4-(4-fluoro-2-methylphenyl)amino)-7-methoxyquinazolin-6-yl)-1-morpholin-1-one (I-c);
[0055]
[0056] Prepared according to the same method as in Example 1, the raw material aniline was replaced by 4-fluoro-2-methylaniline, and the target compound (I-c) was synthesized through 5 steps of chlorination, amination, ester hydrolysis, amidation, and alkylation. 0.118g, yield 77%; m.p.:98.5~100.9℃; IR(v,cm -1 ):3397.98, 3005.33, 2969.50, 2913.54, 1650.50, 1418.90, 1229.24, 997.61, 839.55; 1 H-NMR(600MHz,DMSO-d6):δ9.19(s,1H,NH),8.25(s,1H,C=NH),7.73(s,1H,ArH),7.31(s,1H,ArH) ,7.15(d,J=7.0Hz,2H,ArH),7.05(s,1H,ArH),4.91(s,2H,OCH 2 C=O),3.91(s,3H,OCH 3 ),3.62(s,2H,OCH 2 ),3.56(s,2H,OCH 2 ),3.49(s,2H,NCH 2 ),3.46(s,2H,NCH 2 ),2.14(s,3H,ArCH 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com