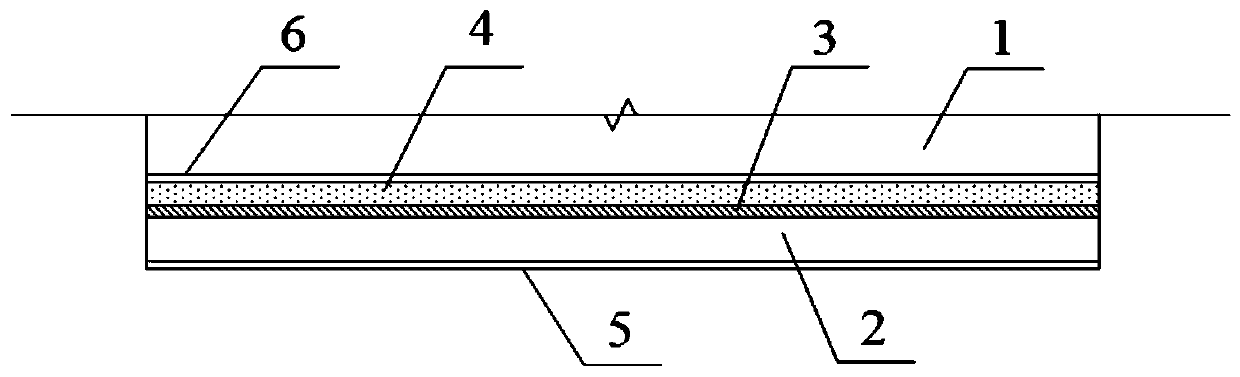
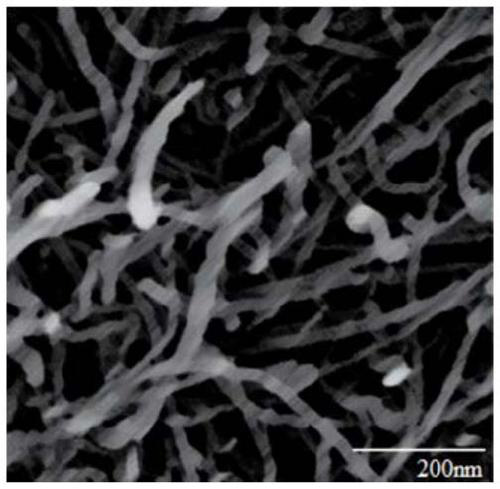
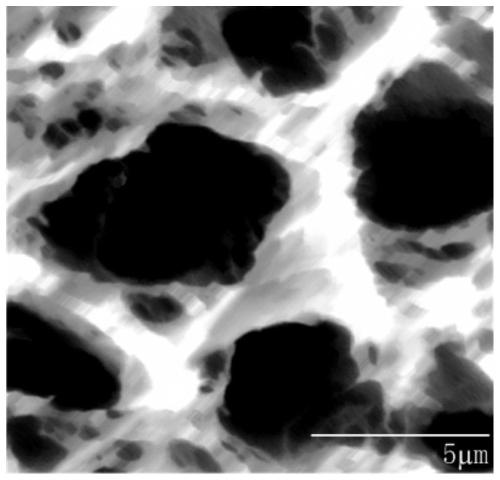
Reusable breathable antibacterial electrode plate and preparation method thereof
An electrode sheet and conductive film technology, applied in the field of biomedical engineering, can solve the problems of increasing the use cost of the electrode sheet, the patient's economic cost, and the large coverage area of the electrode sheet, so as to improve the monitoring accuracy, increase the safety of the operation, and avoid the The effect of contaminating the electrode pads
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The preparation method of the reusable breathable antibacterial electrode sheet in the embodiment of the present invention is specifically carried out according to the following steps:
[0046] S1, 1 part of eugenol and 5 parts of triisopropylsilane are reacted with 0.002 parts of boron trifluoride ether as a catalyst, and triisopropylsilane is used to protect the phenolic hydroxyl group in eugenol to avoid oxidation of the phenolic hydroxyl group;
[0047] According to the mass ratio of eugenol: egg yolk lecithin: β-sitoziol: sodium caseinate is 1: 5: 2.3: 2.3, weigh egg yolk lecithin, β-sitozil and sodium caseinate, and weigh the The reaction product of egg yolk lecithin, β-sitozol, sodium caseinate and eugenol was completely dissolved in 6ml of absolute ethanol at 40°C, and the mixture was quickly injected into the acetic acid buffer solution at a volume ratio of 1:1. The pH value of the acetic acid buffer solution is 6, and the stirring is continued for 40 minutes, ...
Embodiment 2
[0060] The preparation method of the reusable breathable antibacterial electrode sheet in the embodiment of the present invention is specifically carried out according to the following steps:
[0061] S1, 1.1 parts of eugenol and 4.5 parts of triisopropylsilane were reacted with 0.001 part of boron trifluoride ether as a catalyst, and triisopropylsilane was used to protect the phenolic hydroxyl group in eugenol to avoid oxidation of the phenolic hydroxyl group;
[0062] According to the mass ratio of eugenol: egg yolk lecithin: β-sitoziol: sodium caseinate is 1.1: 3: 2.3: 2.5, weigh egg yolk lecithin, β-sitoziol and sodium caseinate, and weigh the The reaction product of egg yolk lecithin, β-sitoziol, sodium caseinate and eugenol was completely dissolved in 6ml of absolute ethanol at 50°C, and the mixture was quickly injected into the acetic acid buffer solution at a volume ratio of 1:1. The pH value of the acetic acid buffer solution is 7, and the stirring is continued for 35...
Embodiment 3
[0073] The preparation method of the reusable breathable antibacterial electrode sheet in the embodiment of the present invention is specifically carried out according to the following steps:
[0074] S1, 1.2 parts of eugenol and 4 parts of triisopropylsilane are reacted with 0.0004 parts of boron trifluoride ether as a catalyst, and triisopropylsilane is used to protect the phenolic hydroxyl group in eugenol to avoid oxidation of the phenolic hydroxyl group;
[0075] According to the mass ratio of eugenol: egg yolk lecithin: β-sitoziol: sodium caseinate is 1.2: 4: 2.3: 2.7, weigh egg yolk lecithin, β-sitoziol and sodium caseinate, and weigh the The reaction product of egg yolk lecithin, β-sitozol, sodium caseinate and eugenol was completely dissolved in 4ml of absolute ethanol at 60°C, and the mixture was quickly injected into the acetic acid buffer solution at a volume ratio of 1:1. The pH value of the acetic acid buffer solution is 7, and the stirring is continued for 30 mi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com