Therapeutic and diagnostic methods for cancer
A kind of therapy, cancer technology, applied in chemical instruments and methods, medical automated diagnosis, biochemical equipment and methods, etc., can solve problems such as inappropriate health conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0569] Embodiment 1. Method
[0570] A blood-based assay was used to assess the association between clinical response to treatment with atezolizumab (MPDL3280A) and hematological tumor mutational burden (bTMB) score in patients with non-small cell lung cancer (NSCLC) who Two clinical trials, Phase II clinical trial POPLAR (Clinical Trial ID No: NCT01903993) and Phase III clinical trial OAK (Clinical Trial ID No: NCT02008227), in which atezolizumab was administered as monotherapy, were enrolled.
[0571] Research design
[0572] Pre-treatment blood samples from patients with NSCLC enrolled in the POPLAR and / or OAK studies in which atezolizumab was administered as monotherapy were assessed for bTMB score and / or maximum somatic allele frequency (MSAF).
[0573] The POPLAR (Clinical Trial ID: NCT01903993) patient population evaluated for the bTMB score consisted of 273 patients. Patients are eligible for inclusion in the POPLAR study if they have: locally advanced or metastatic ...
Embodiment 2
[0631] Example 2. Analysis of the Association Between bTMB Score and Clinical Response to Atezolizumab Treatment in Patients with NSCLC
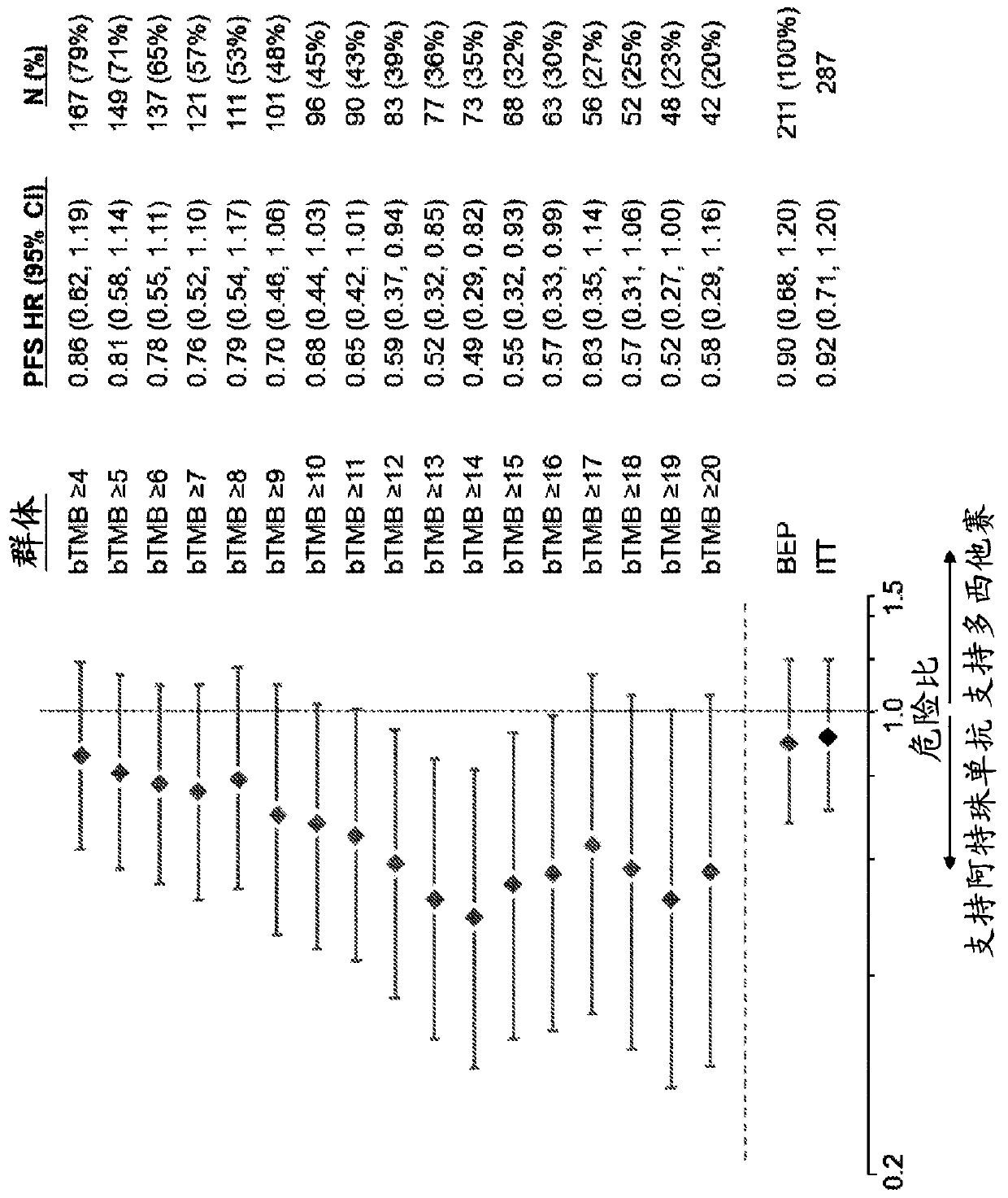
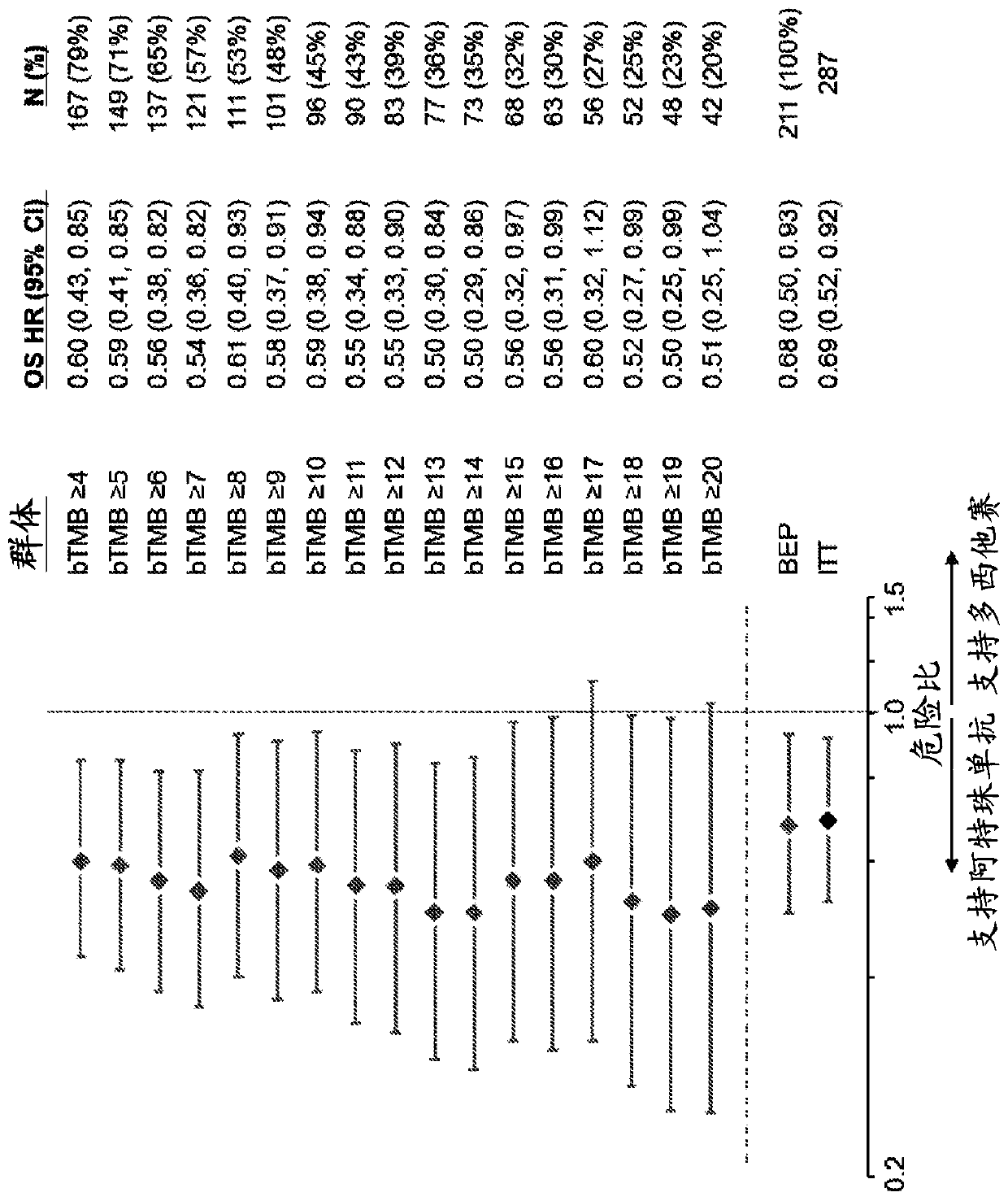
[0632]To assess whether the bTMB score could be used as a predictive biomarker of patient response to atezolizumab treatment, bTMB score was assessed in pre-treatment blood samples obtained from patients in the POPLAR or OAK trials as described in Example 1. Overall survival (OS) and progression-free survival from the POPLAR and OAK trials were observed in patients with a positive diagnosis (Dx+) based on a bTMB score equal to or higher than the reference score of ≥4 to ≥20 and ≥4 to ≥26, respectively Period (PFS) ( Figure 1A , Figure 1B , Figure 2A with Figure 2B ). In POPLAR, PFS and OS benefits were observed at all bTMB score cutoffs between ≥10 and ≥20 (eg, between ≥12 and ≥20) ( Figure 1A with Figure 1B ). In both the POPLAR and OAK studies, Dx+ patients with a bTMB score greater than or equal to a reference bTMB score of 18 ...
Embodiment 3
[0653] Example 3: bTMB is independent of PD-L1 IHC and histology
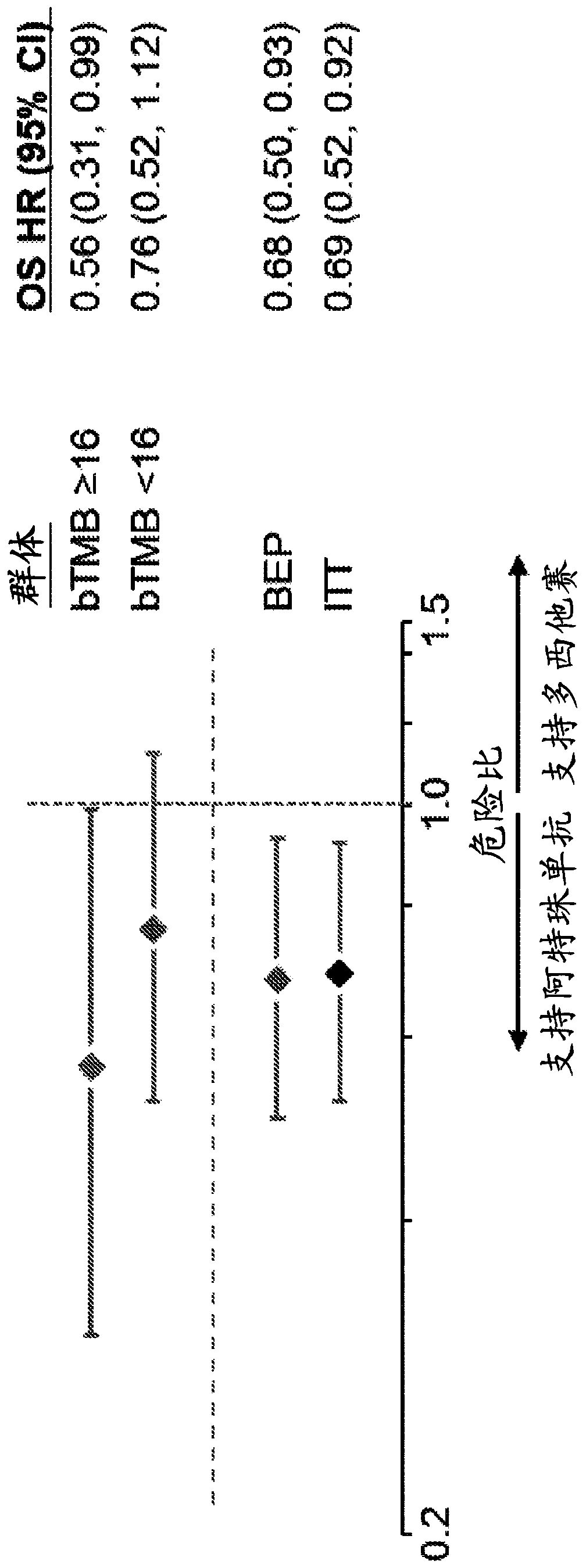
[0654] Tables 10 and 11 show the clinical characteristics of the bTMB subgroup in OAK. Within OAK BEP, positive bTMB status (≥16) was associated with smoking (P=1.3e-10), which was associated with substantial mutagen exposure, SLD of target lesions at baseline (P=4.8e-08), metastatic sites The number (P=0.0055) is consistent with the expression of PD-L1 (TC1 / 2 / 3 or IC1 / 2 / 3 (P=0.0062)) (Table 10). Baseline characteristics were balanced between treatment groups above a bTMB cutpoint of > 16 (Table 11). Mean bTMB values were 11.22 (95% CI: 10.09, 12.36) in patients with non-squamous histology and 12.4 (95% CI: 11, 13.8) in those with squamous histology ( Figure 10 ).
[0655] Table 10. Characteristics of the subgroup with bTMB ≥ 16 in the OAK study
[0656]
[0657]
[0658] TC0 and IC0, <1% of TC and IC express PD-L1; TC1 / 2 / 3 or IC1 / 2 / 3, ≥1% of TC or IC express PD-L1; TC2 / 3 or IC2 / 3, ≥5 % of TC or IC ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com