A pharmaceutical composition comprising a probiotic and a prebiotic to prevent acquisition of or treat drug resistant infections
A technology of composition and probiotics, applied in the direction of drug combination, anti-infective drug, pharmaceutical formula, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0057] According to a preferred embodiment of the present invention, the prebiotics include one or more of the following: (a) oligosaccharides, (b) fructooligosaccharides ("FOS"), such as soybean fructooligosaccharides, inulin or banana Fiber, (c) pectin or pectin polysaccharide, (d) mannan, such as guar gum, locust bean gum, konjac or xanthan gum, (e) pentosan, beta-glucan, arabican Sugars and galactans, such as larch arabinogalactan, and (f) mixtures thereof.
[0058] According to one embodiment, the pharmaceutical composition of the present disclosure may be administered in a solid, semi-solid or liquid oral dosage form. In one embodiment of the present disclosure, the pharmaceutical formulations may be in the form of emulsions, solutions, suspensions, syrups, elixirs, tablets, capsules, pills, granules and suppositories. In another embodiment of the present disclosure, the pharmaceutical formulation can be water dispersible granules (WG), suspension concentrates (SC), wet...
Embodiment
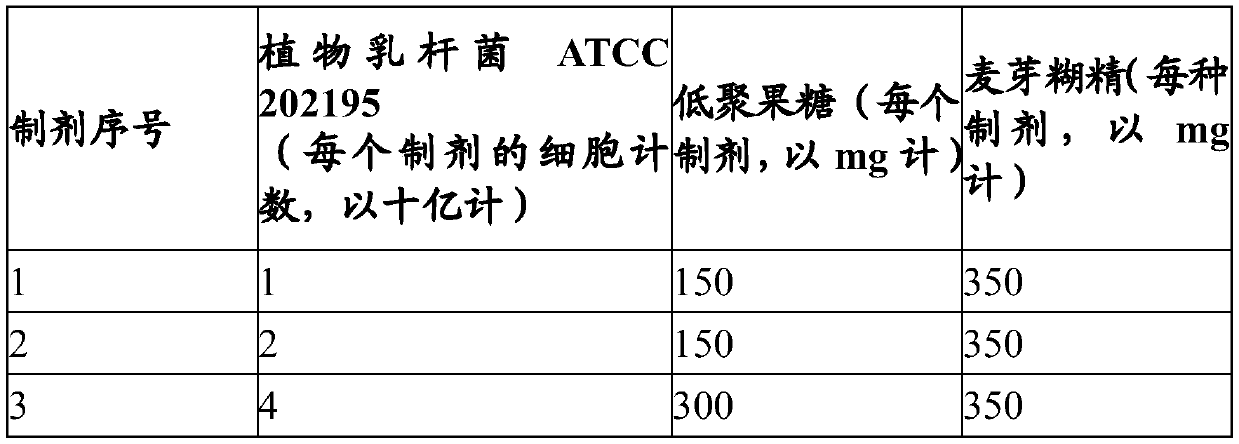
[0095] Composition comprising Lactobacillus plantarum ATCC 202195 and fructooligosaccharides
[0096] Powder formulations as shown in Table 1 below were prepared, each formulation including Lactobacillus plantarum ATCC 202195, fructooligosaccharide and maltodextrin.
[0097] Table 1: Powder Formulations
[0098]
[0099] Each of the above formulations 1 to 3 was packaged in a sachet and stored in a cool, moisture-proof container for further use.
[0100]Administration of Lactobacillus plantarum ATCC 202195 and fructooligosaccharides to infants
[0101] A cohort of infants was born orally administered a synergistic biologic containing Lactobacillus plantarum ATCC 202195 and fructooligosaccharides. Newborns were recruited from over 70 community sites and two tertiary care centers in Odisha, India - Capital Hospital, Bhubaneswar and SCB Medical College, Cuttack. All infants were more than 35 weeks' gestation and weighed more than 1800 grams at birth. The group designated a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com