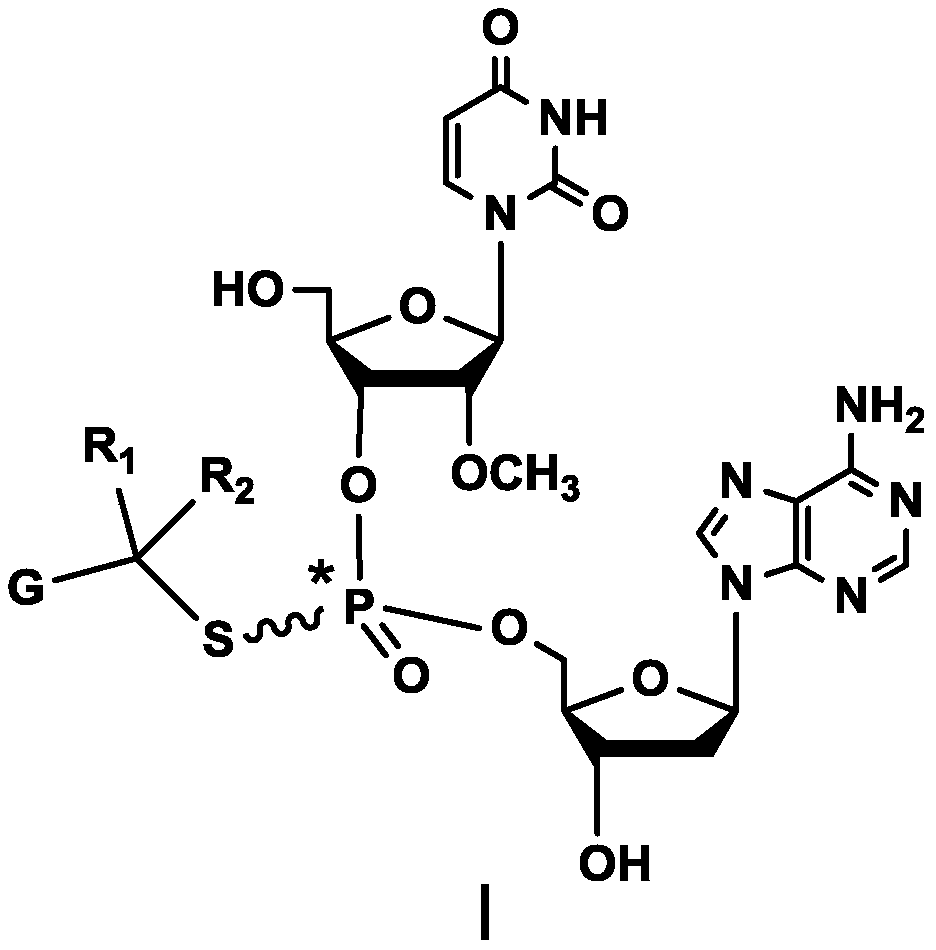
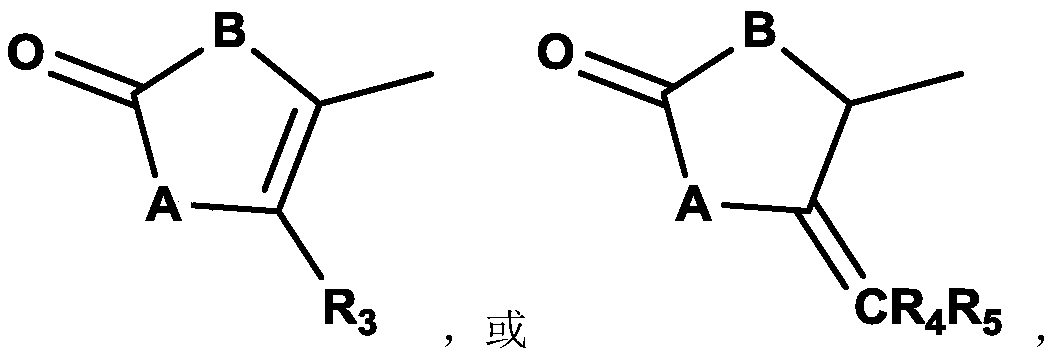
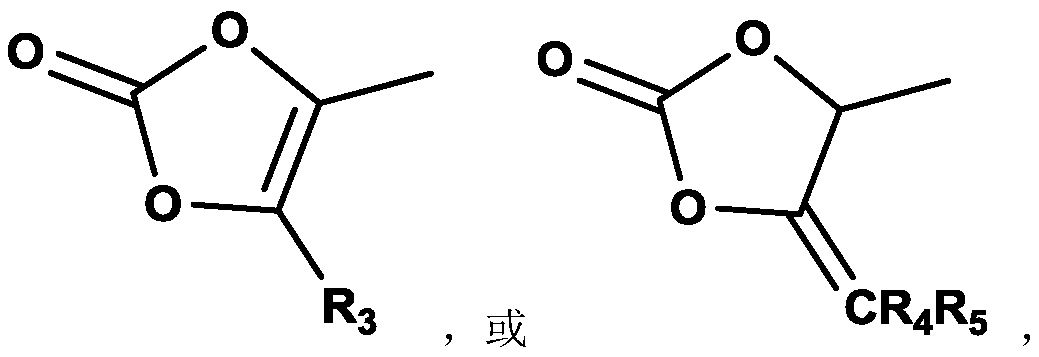
Compound containing dinucleotide structure
A compound and selected technology, applied in the field of compounds containing dinucleotide structures, can solve problems such as not meeting the Lipinski standard
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0110] The synthetic method of embodiment 1 compound BG002:
[0111]
[0112] Under nitrogen protection, in the reaction flask, SM3 (4.5g, 7.7mmol), DMF (100ml), diisopropylethylamine (1.1g, 8.5mmol), sodium iodide (0.58g, 5.8mmol), 4 -Chloromethyl-5-methyl-1,3-dioxol-2-one (1.2g, 8.4mmol), after the addition was completed, the reaction was stirred at room temperature overnight, and the reaction solution was purified by column chromatography to obtain BG002 2.0g, purity 98.08%, yield 57.3%.
[0113] MS Calcd: 699; MS Found: 700 [M+H] + .
[0114] 1 H-NMR (DMSO-d 6 ,400Hz):
[0115] δ2.0177-2.0411(d,3H); 2.3059-2.3700(m,1H); 2.8201-2.9104(m,1H); 3.33(s,3H); 3.5887(s,2H); ); 4.0326-4.1349(m,3H); 4.1831-4.2859(m,1H); 4.3294-4.4024(m,1H); (t,1H);5.5369-5.5509(dd,1H);5.7020-5.7325(m,1H);5.8897-5.9135(m,1H);6.3617-6.3993(m,1H);7.2728(s,2H);7.8637 -7.8938 (m, 1H); 8.1423 (s, 1H); 8.2848-8.2965 (d, 1H); 11.4335 (s, 1H).
[0116] 13 C-NMR (DMSO-d 6 ,400Hz):
[0117] δ9.1...
Embodiment 2
[0129] The synthetic method of embodiment 2 compound BG004:
[0130]
[0131]
[0132] (1) Preparation of BG004-03:
[0133] N 2 protection, at 0°C, add acetonitrile (420ml), BG004-02 (12.0g, 51.3mmol), p-toluenesulfonyl azide (12.3g, 51.3mmol) to the reaction flask in sequence, and then add triethylamine (21.4ml, 15.4 mmol), maintained at 0°C, stirred for 30 min, and then reacted at room temperature for 4 h. The reaction solution was concentrated to dryness under reduced pressure, and slurried with 150ml×2 diethyl ether / petroleum ether=2:1 mixed reagent. Suction. The filtrate was concentrated to dryness under reduced pressure, and purified by column chromatography with ethyl acetate / n-hexane mixed solvent to obtain 12.79 g of oily substance BG004-03 with a yield of 96%.
[0134] (2) Preparation of BG004-04:
[0135] Add tetrahydrofuran (250ml), purified water (120ml), BG004-03 (12.7g, 48.7mmol), Rh(OAc) 4 (165mg, 0.37mmol), reflux reaction for 5h. After the react...
Embodiment 3
[0148] The synthetic method of embodiment 3 compound BG012:
[0149]
[0150] (1) Preparation of BG012-02:
[0151] Under nitrogen protection, hydroxyacetone (21.0 g, 280 mmol) and dichloromethane (200 ml) were added to the reaction flask. The temperature was lowered to 0°C, and triphosgene (30.0 g, 110 mmol) was added. Control the temperature to ≤-8°C, and add N,N-dimethylaniline (37.0 g, 300 mmol) dropwise. Control the temperature at 0°C, stir and react for 15 minutes, and then react at room temperature for more than 2 hours. The temperature of the reaction solution was cooled to 5°C, and the solution was successively washed with ice-cold 3M dilute hydrochloric acid (40ml), water (30ml), and saturated sodium chloride (30ml). The organic layer was dried by adding anhydrous magnesium sulfate, filtered, concentrated under reduced pressure to 1 / 2 volume, and refluxed for 3h. Afterwards, it was concentrated to dryness to obtain an oily substance, which was heated to 170° C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com