Nano material for osteoclast acidic closed region and preparation method thereof
A technology of nanomaterials and osteoclasts, applied in medical preparations with non-active ingredients, medical preparations containing active ingredients, bone diseases, etc., to improve drug utilization, improve trabecular bone gap, and reduce side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
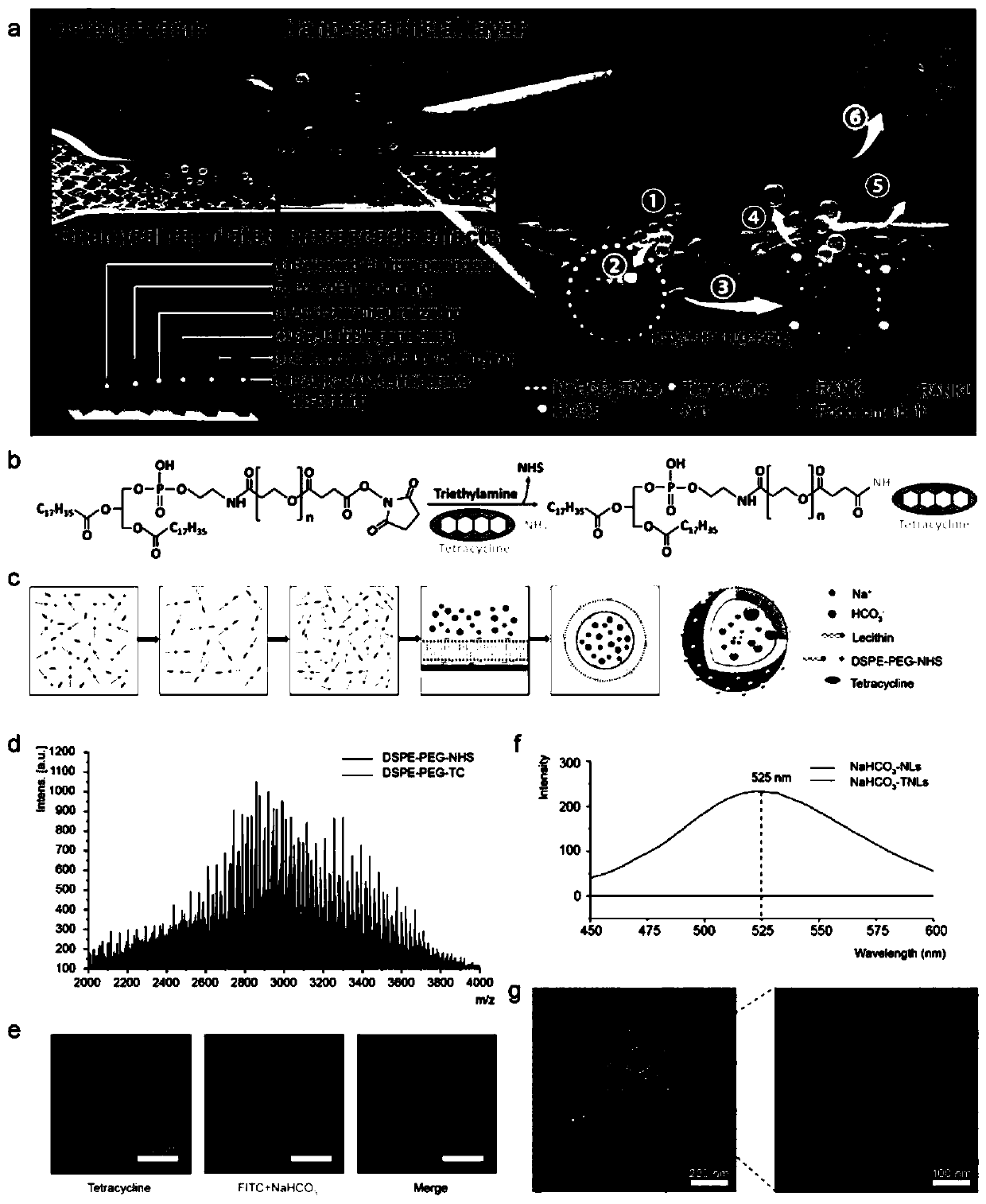
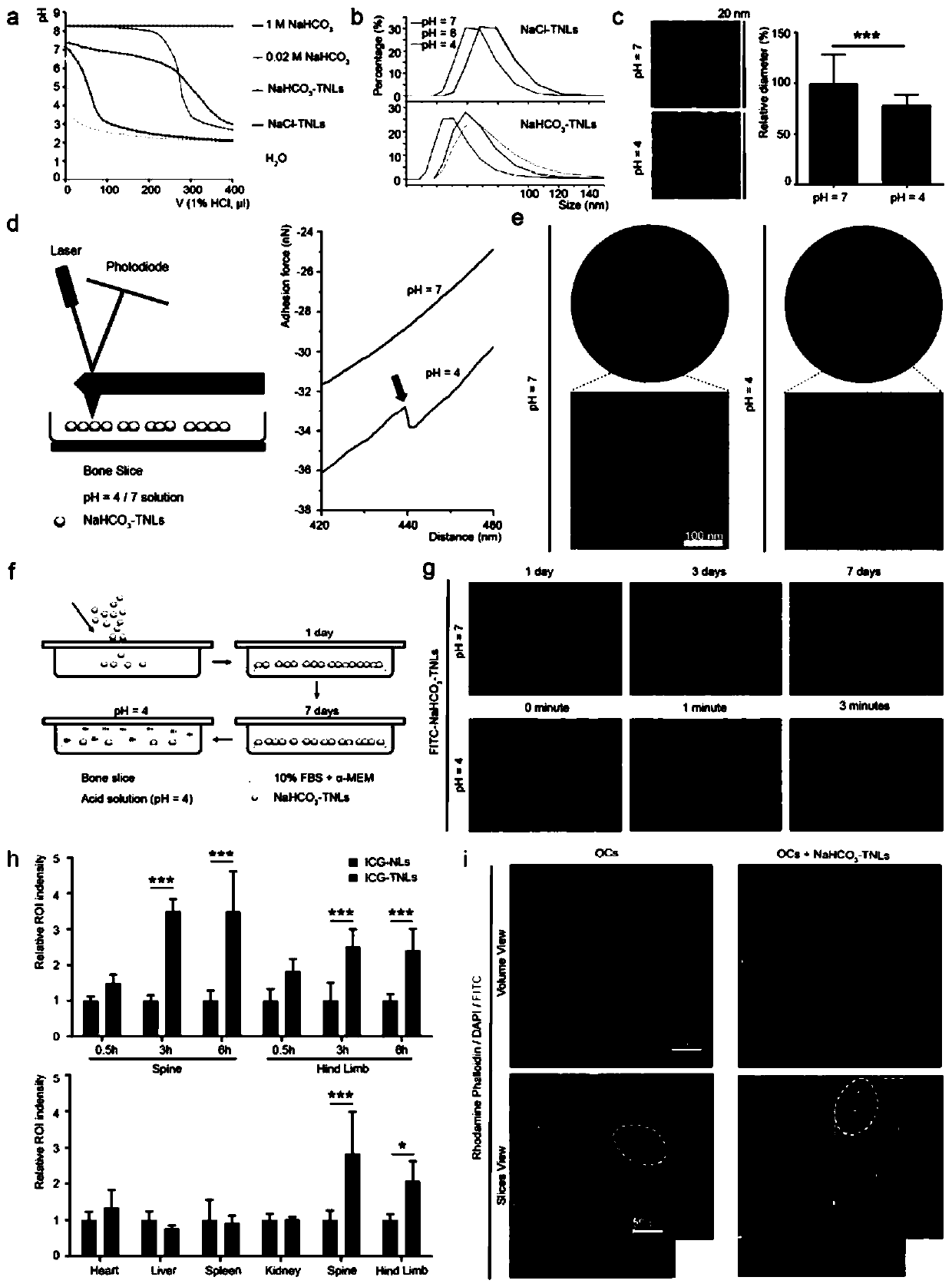
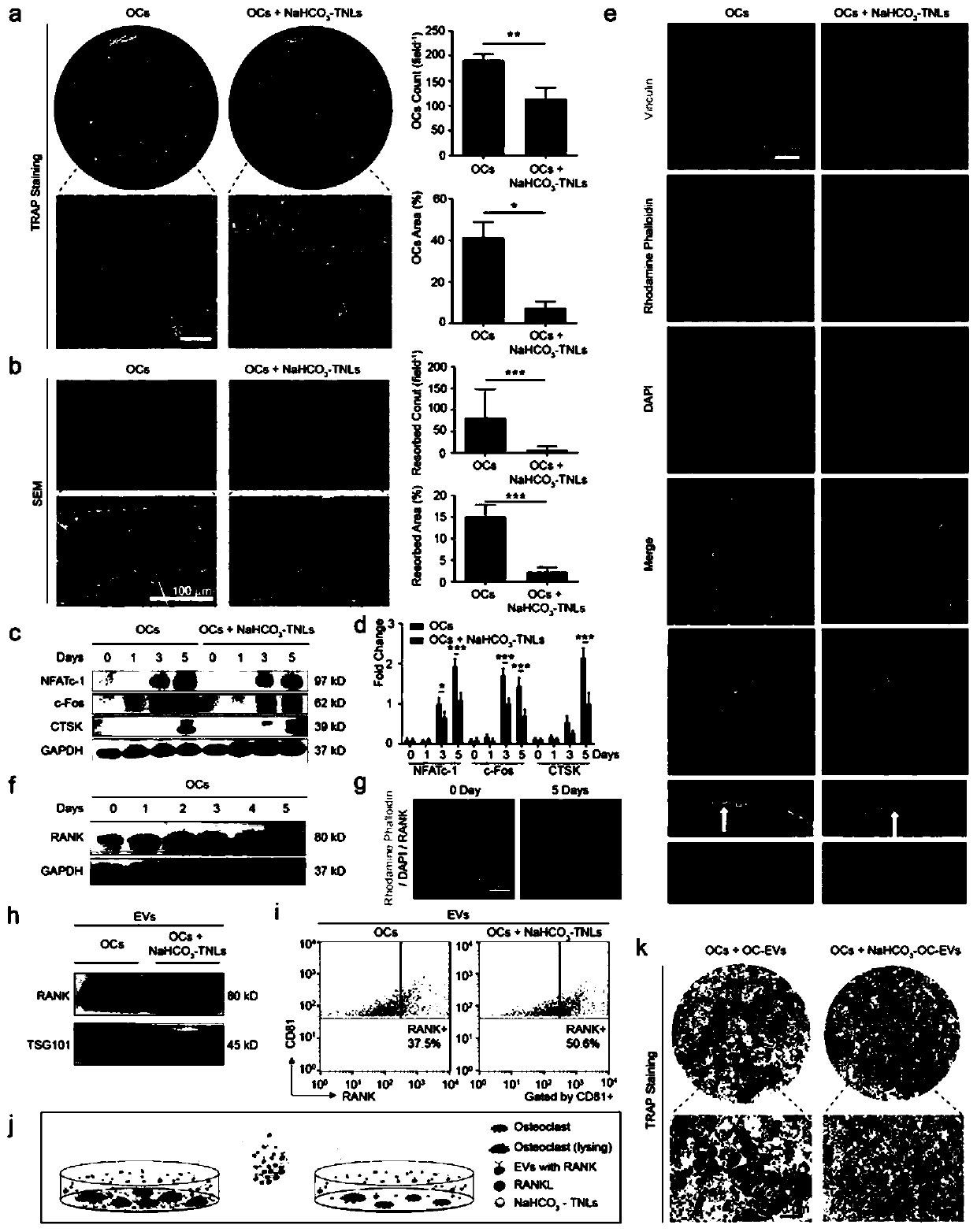
[0032] Embodiment 1, preparation of tetracycline-modified nanoliposomes loaded with sodium bicarbonate
[0033] 1. Dissolve 20.00 mg of DSPE-PEG-NHS and 3.05 mg of tetracycline in 10.00 mL of chloroform, add triethylamine to adjust the pH to 8.2, and cross-link with magnetic stirring at room temperature for 48 hours.
[0034] 2. Dissolve the product obtained in step 1, 100.00 mg lecithin, and 16.00 mg cholesterol in chloroform, and make a thin film in a rotary evaporator.
[0035] 3. Add 10mL of 1mol / L sodium bicarbonate solution into the flask for hydration by shaking.
[0036] 4. Perform phacoemulsification. The phacoemulsification process is 2s on, 3s off, 40% power, and 10 minutes
[0037] 5. Dialyze in the dialysis bag for 72 hours, take it out and filter it through a 0.22 micron filter head, and store it at 4 degrees.
Embodiment 2
[0038] Example 2, preparation of alendronic acid-modified nanoliposomes loaded with sodium bicarbonate
[0039] 1. Dissolve 20.00 mg of DSPE-PEG-NHS and 2.30 mg of alendronate sodium in 10.00 mL of chloroform, add triethylamine to adjust the pH to 8.2, and cross-link with magnetic stirring at room temperature for 48 hours.
[0040] 2. Dissolve the product obtained in step 1, 100.00 mg lecithin, and 16.00 mg cholesterol in chloroform, and make a thin film in a rotary evaporator.
[0041] 3. Add 10mL of 1mol / L sodium bicarbonate solution into the flask for hydration by shaking.
[0042] 4. Perform phacoemulsification. The phacoemulsification process is 2s on, 3s off, 40% power, and 20 minutes
[0043] 5. Dialyze in the dialysis bag for 72 hours, take it out and filter it through a 0.22 micron filter head, and store it at 4 degrees.
Embodiment 3
[0044] Embodiment 3, preparation of tetracycline-modified nanoliposomes loaded with potassium bicarbonate
[0045] 1. Dissolve 20.00 mg of DSPE-PEG-NHS and 3.05 mg of tetracycline in 10.00 mL of chloroform, add triethylamine to adjust the pH to 8.2, and cross-link with magnetic stirring at room temperature for 24 hours.
[0046] 2. Dissolve the product obtained in step 1, 80 mg lecithin, and 16.00 mg cholesterol in chloroform, and make a thin film in a rotary evaporator.
[0047] 3. Add 10mL of 1mol / L potassium bicarbonate solution to the flask for hydration by shaking.
[0048] 4. Perform phacoemulsification. The phacoemulsification process is 2s on, 3s off, 40% power, and 10 minutes
[0049] 5. Dialyze in the dialysis bag for 72 hours, take it out and filter it through a 0.22 micron filter head, and store it at 4 degrees.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com