Tumor individualized specific therapeutic vaccine and preparation method thereof
A technology for therapeutic vaccines and tumor patients, applied in the direction of anti-tumor drugs, antibody medical ingredients, medical preparations containing active ingredients, etc., can solve the problem of efficient expansion and maturation of DC cells, difficult induction of CTL, and patients Long waiting time and other problems, to achieve the effect of shortening the waiting time, outstanding treatment effect, and short treatment cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
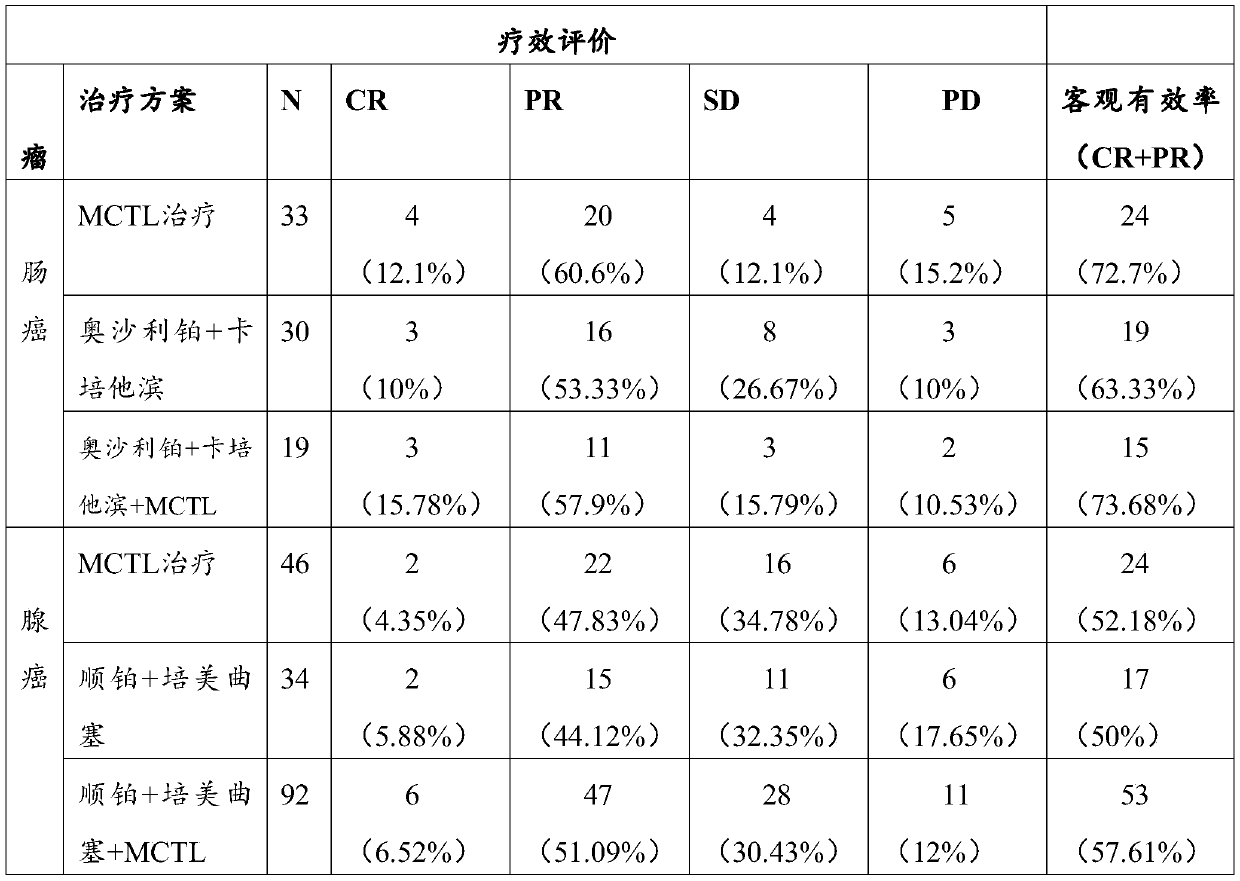
Image
Examples
Embodiment 1
[0050] Example 1: Molecular target detection, analysis and synthesis
[0051] 1. Sample collection: collect 5ml of blood sample to be tested according to clinical blood collection requirements, separate serum, and keep at 2-8°C
[0052] Transportation, ensure delivery to the laboratory within 72 hours.
[0053] 2. Virus detection and sample retention:
[0054] 3. Enrichment: add the serum to be tested into phosphoric acid solution and acetonitrile solution, the volume ratio of serum, phosphoric acid and acetonitrile is 1:1-2:4-6; mix well and centrifuge to get the supernatant;
[0055] 4. Purification: add acetonitrile solution to the supernatant and mix evenly, acetonitrile: supernatant volume ratio is 0.3-1:2, centrifuge to get the supernatant and concentrate to 1-50 μl, then add water to dilute;
[0056] 5. Collection: filter through a hydrophobic solid phase extraction column Oasis Prime HLB, wash the solid phase extraction column with methanol, elute with a mixed soluti...
Embodiment 2
[0060] Example 2: In vitro amplification and activation method for tumor-specific dendritic cell targets
[0061] Blood sample collection: Collect 80--120ml of peripheral blood from patients according to clinical blood collection requirements, transport at 2-8°C, and ensure delivery to the laboratory within 6 hours.
[0062] Separation of PBMCs: Mononuclear cells (PBMCs) in blood were separated by density gradient centrifugation with lymphocyte separation medium.
[0063] Culture of DC cells and MCTL cells: According to the results of PBMC counting, adjust the cells to about 1×10 with culture medium 7 cells / ml, inoculate, and incubate in a carbon dioxide incubator at 37°C; 5% CO 2 ; Incubation for 30min.
[0064] Culture the adherent cells after incubation as DC: take out the culture flask, shake gently to float the suspended cells that settled at the bottom, and transfer to a centrifuge tube. After adhering to the wall, wash repeatedly with normal saline several times unti...
Embodiment 3
[0068] Example 3: In vitro amplification and activation method of tumor-specific dendritic cell (DC) target
[0069] 1. Specimen collection:
[0070] The blood picture of melanoma patients is within the normal range (WBC: 4-10×10 9 , LYM%: 20%-40%), the fluctuation does not exceed 5%, the circulation volume of peripheral blood by machine sampling is 1000-4000ml, the number of mononuclear cells: more than 1×10 9 . Blood drawing method 50-100ml, the number of mononuclear cells: more than 1×10 6 .
[0071] 2. Isolation of Mononuclear Cells
[0072] Transfer the PBMC cell suspension sample, pour it into a centrifuge tube and centrifuge, balance the centrifuge tube, centrifuge at 1310g for 10min, RT. The plasma layer was aspirated and discarded. Dilute the above cell pellet with normal saline at a ratio of 1:1 and mix by pipetting. Slowly add 30ml of the diluted cell suspension into a 50ml sterile centrifuge tube containing 15ml of human lymphocyte separation medium at room ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com