Plant fermentation product and use thereof for regulating gene expression and cardiovascular care
A plant fermentation and plant extract technology, applied in the field of regulating gene expression and cardiovascular health care, and plant fermentation products, can solve problems such as harmfulness to human health and high product prices.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1. Preparation of plant fermented product
[0038] First, mix Gardenia jasminoides, Portulacaoleracea and water from China at a volume ratio of 1-5:1:40-60, and sterilize and extract 0.5- For 3 hours, a botanical extract is obtained. The plant extract was cooled to room temperature for subsequent three-stage fermentation. The three-stage fermentation is to inoculate plant extracts with 0.01-0.5% (v / v) Saccharomycescerevisiae BCRC 20271 and 0.01-o.25% (v / v) Lactobacillus plantarum TCI028 (BCRC) in sequence. 910805) fermented for 1 to 5 days; 1 to 20% (v / v) acetic acid bacteria (Acetobacter aceti) BCRC11688 (the above bacterial strains were all purchased from Taiwan's Food Industry Research and Development Institute (FIRDI) biological Resource Conservation and Research Center (Biosource Collection and Research Center, BCRC)) fermented for 3-8 days. Thereafter, it was concentrated under reduced pressure at 45°C to 70°C, and filtered through a 200 to 400 mesh ...
Embodiment 2
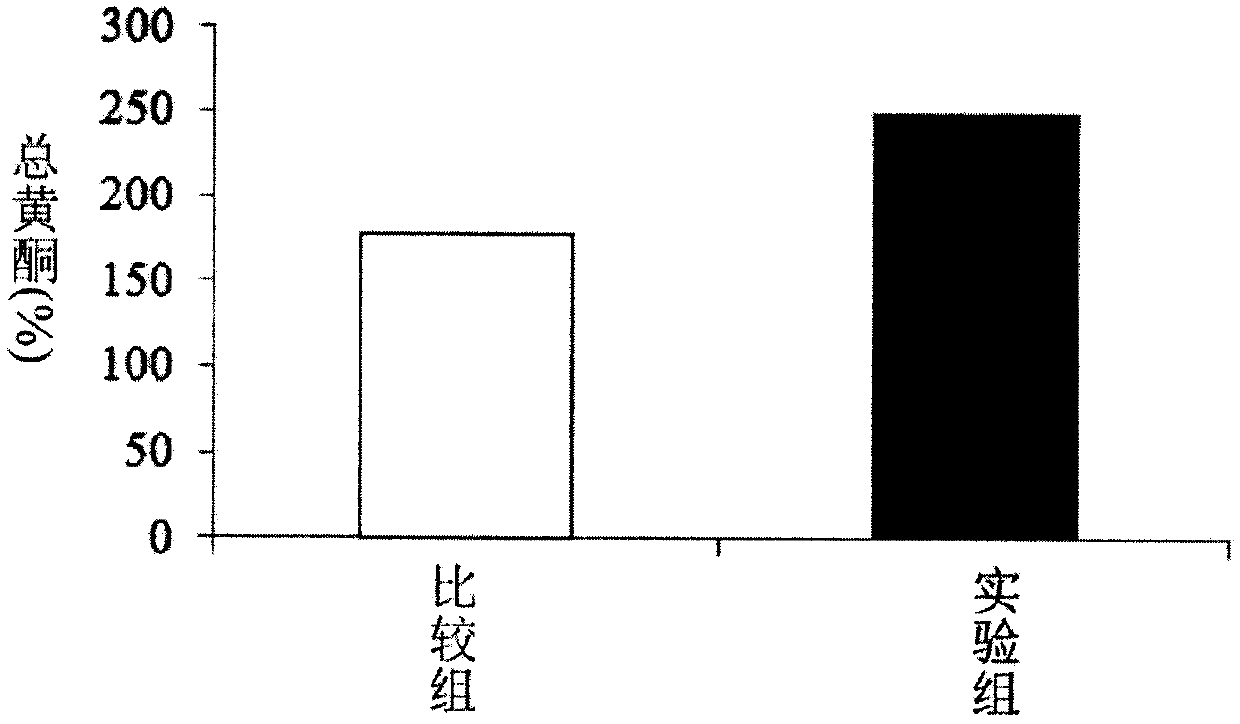
[0039] Example 2. Detection of total flavonoid content of plant fermented product
[0040] The experimental process for detecting the total flavonoid content is as follows: the detection of the total flavonoid content is represented by the equivalent of rutin (ChromaDexASB-00018440) as the relative content of the total flavonoid. The preparation materials contained 10% Aluminum nitrate (Aqueous solution) (Alfa Aesar 12360), 5% Sodium citrate (Sodium nitrite) (Aqueous solution) (Sigma31443), 4% Sodium hydroxide (Sodium hydroxide) (Aqueous solution) (Macron 7708-10) and 200μg / mL rutin (methanol solution).
[0041] Take 0, 200 μL, 400 μL, 600 μL, 800 μL, 1000 μL and 1200 μL of the above-mentioned rutin standard solution and add them to the test tubes respectively, add 1200 μL, 1000 μL, 800 μL, 600 μL, 400 μL, 200 μL and 0 μL of water in sequence, shake and mix evenly; take 200 μL Rutin solutions of various concentrations; add 200 μL of 5% sodium citrate, mix well and let stand f...
Embodiment 3
[0044] Example 3. Evaluation of the effectiveness of plant fermented products on cardiovascular health care
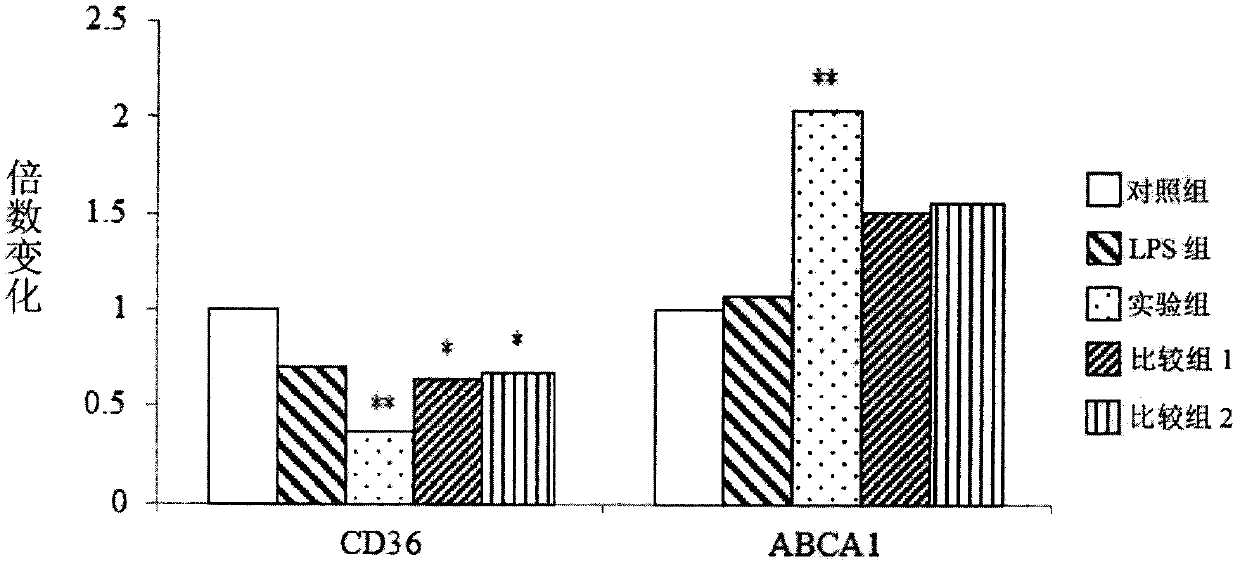
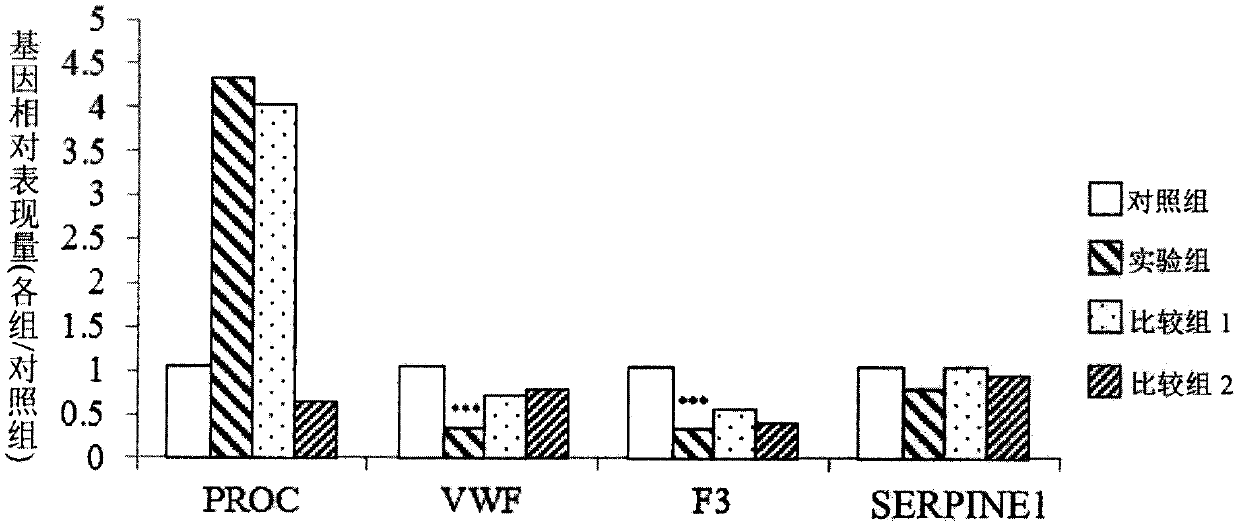
[0045] 3.1 Efficacy of plant fermented products in regulating gene expression related to atherosclerosis
[0046] First, human monocytic cell (human monocytic cell) THP-1 (corresponding to ATCC, TIB202) was cultured in RPMI-1640 medium (Gibco) (added with 10% fetal bovine serum (fetal bovine serum, FBS) (Gibco ), 0.05mM 2-mercaptoethanol (2-mercaptoethanol), 100 units / mL penicillin (penicillin) and 100μg / mL streptomycin (streptomycin)). Add 2 mL of culture medium to each well of the 6-well culture plate, so that each well has 1.5 x 10 5 THP-1 cells. Next, a differentiation medium with 500 nM phorbol 12-myristate 13-acetate (PMA) was added and cultured for 48 hours. Afterwards, the medium was replaced with fresh medium and cultured for another 48 hours.
[0047] In addition, prepare a single plant fermented product (comprising a single purslane fermented product, a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com