Immortalized car-t cells genetically modified to eliminate t-cell receptor and beta2-microglobulin expression
A cell receptor and immortalization technology, applied in the field of immunotherapy, can solve problems such as death, tissue damage, and limit the efficacy of transferred cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0074] The chimeric antigen receptor (CAR) is designed for adoptive immunotherapy by connecting the extracellular antigen binding domain with the transmembrane domain and the intracellular signal domain (internal domain). It is a useful anti-tumor method for eradicating tumor cells by adoptively transferring T cells expressing chimeric antigen receptors to recognize specific antigens present on tumor cells and activate T cells to specifically lyse these tumor cells. The key aspect of this CAR strategy is the selection of target epitopes that are specifically or selectively expressed on tumors, are present on all tumor cells, and are membrane epitopes that are not easily detached from the cell surface or regulated. . However, ideally, CAR-T cells will be able to be used as general reagents or drugs suitable for any mammalian (such as human) recipient. In order to use cells in this way, one must prevent them from being rejected in the graft-versus-host response without compromis...
Embodiment 1
[0246] Example 1: Preparation of TALL-104 cells for electroporation
[0247] Divide exponentially growing TALL-104 cells to 0.7×10 6 Cells / mL were seeded in complete TALL-104 cell culture medium [Myelocult H5100 medium (StemCell Technologies 05150); 1% sodium pyruvate (Invitrogen 11360-070); 1% non-essential amino acids (Invitrogen 11140-050); 4uM Hydrocortisone (StemCell Technologies 07904); 100IU / ml recombinant human IL-2 (R&D Systems 202-IL, 2.1E4 IU / ug)] and incubate at 37°C. On the next day, collect the required number of cells (1×10 6 / Electroporation). The cells were washed twice with 10 mL of cold Opti-MEM (ThermoFisher Scientific, 31985062), centrifuged at 100×g for 10 min and resuspended in 0.1 mL×(total number of electroporation experiments+1) previously equilibrated to room temperature OPTI-MEM.
Embodiment 2
[0248] Example 2: Preparation of ribonucleoprotein complex
[0249] Guide RNA
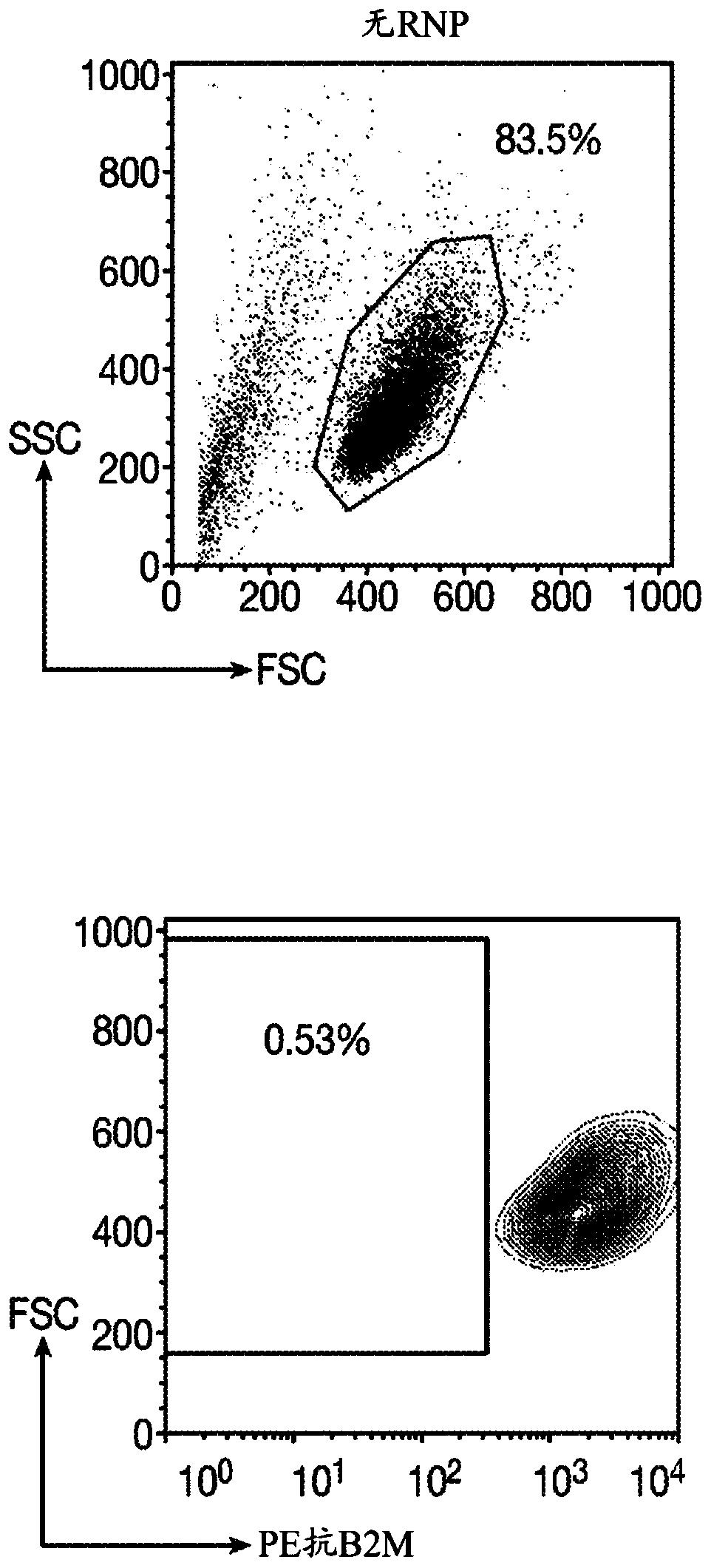
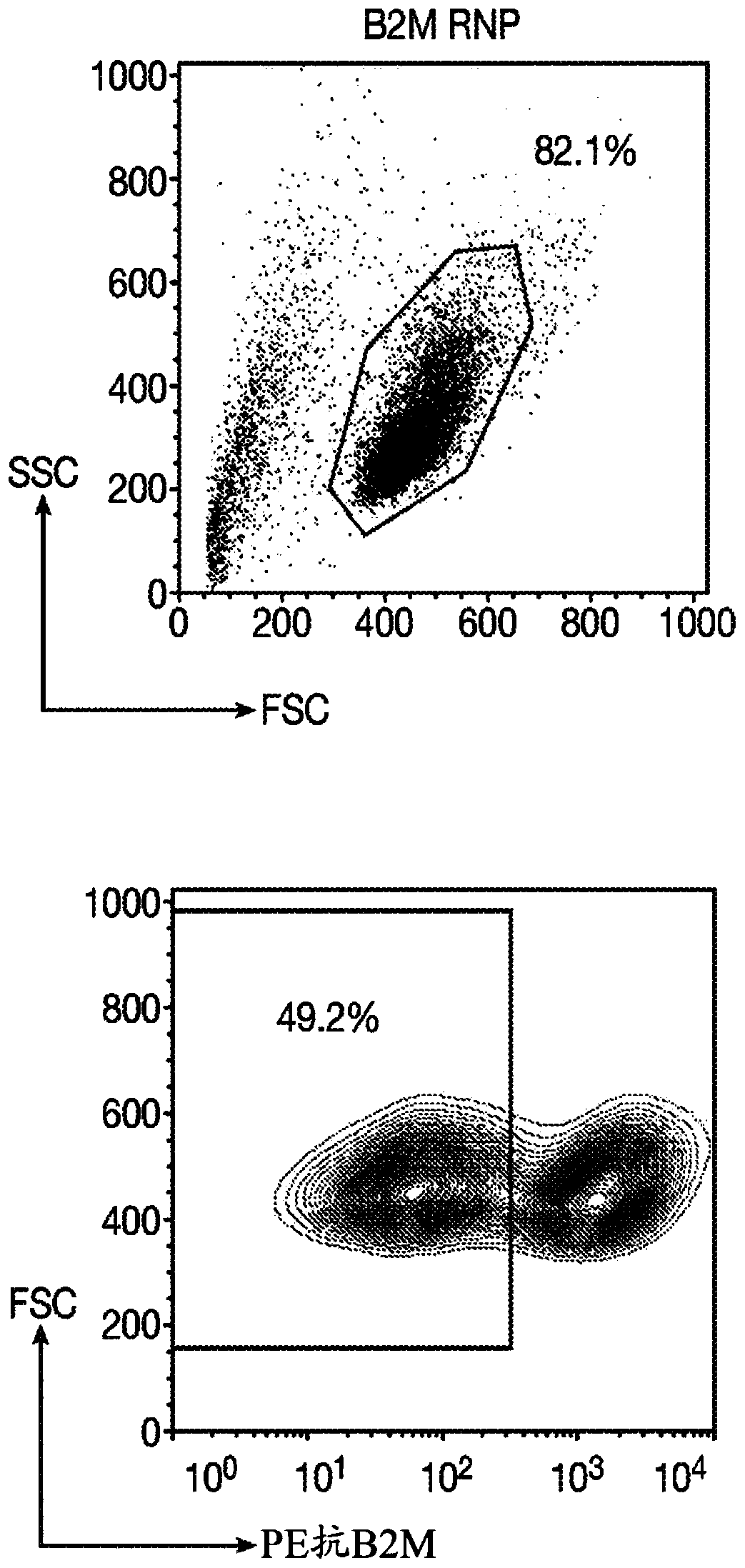
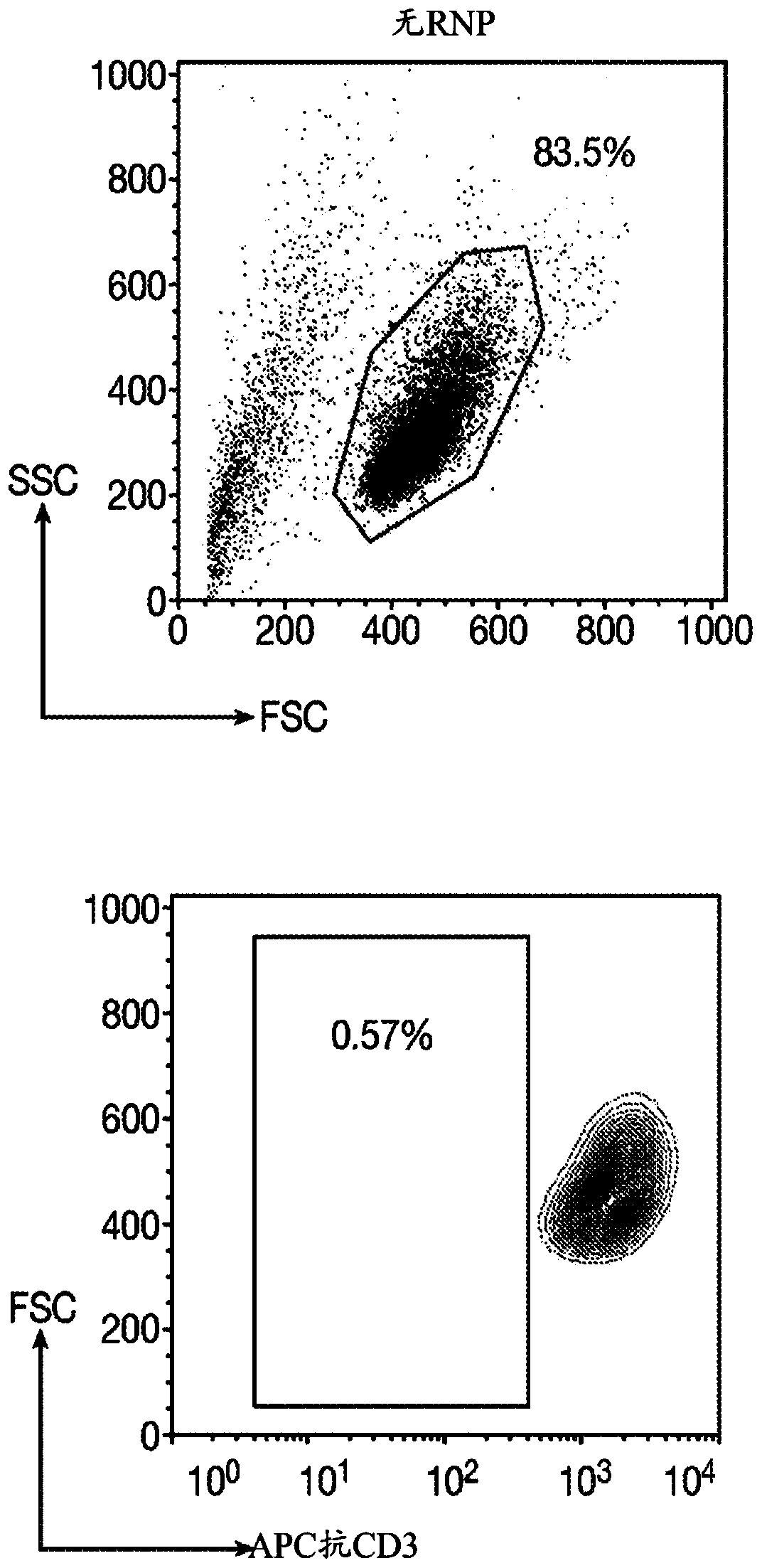
[0250] A gRNA is designed to target the first exon of the constant chain of the TCRα gene (TRAC). The targeted sequence located upstream of the TCRα transmembrane domain is necessary for TCRα and β to assemble and address the cell surface. After Cas9 endonuclease-mediated DNA cleavage, non-homologous end joining (NHEJ) or the integration of CAR through homology-directed repair (HDR) will cause the ablation of the TRAC gene. In order to disrupt the B2M locus, a gRNA targeting the first exon was designed. For the gene KIR3DL2, which is responsible for the production of transmembrane glycoproteins on natural killer cells and T cell subsets, gRNA targeting the third exon was designed.
[0251] Table 1: Sequence of guide RNA used for gene editing .
[0252]
[0253] gRNA: tracrRNA duplex formation
[0254] Customized synthesis of target specific Alt-R by Integrated DNA Technologies TM CRISPR-Cas9 guide R...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com