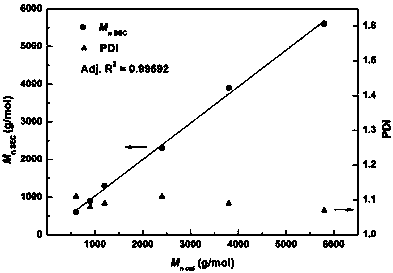
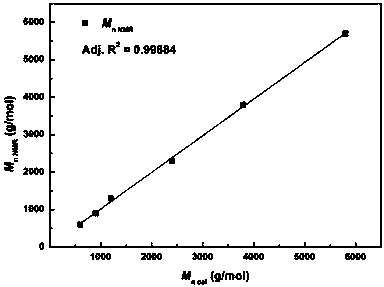
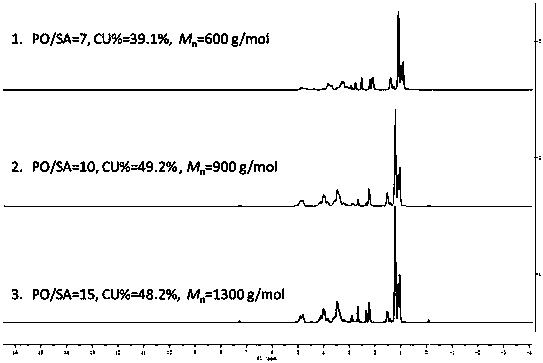
Active hydrogen tolerant catalyst, preparation method thereof and ultralow-molecular-weight poly(carbonate-ether) polyol
A catalyst and active hydrogen technology, applied in the field of polymers, can solve the problems of catalyst deactivation, uncontrollable polymerization reaction, poor catalyst proton tolerance, etc., and achieve the effect of high proton tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0063] The present invention provides a preparation method of the active hydrogen tolerant catalyst shown in the formula (I), comprising:
[0064] A) react p-hydroxybenzaldehyde, substituted benzaldehyde, and pyrrole under the condition of propionic acid reflux, and separate by column chromatography to obtain a monohydroxyl-substituted asymmetric porphyrin;
[0065] Maleimide and halogenated alkyl alcohol carry out dehydration reaction under the condition of diisopropyl azodicarboxylate and triphenylphosphine to obtain the maleimide substituted by halogenated alkyl chain,
[0066] B) reacting the maleimide substituted with the haloalkyl chain and the monohydroxyl-substituted porphyrin to synthesize an ether under alkaline conditions to prepare a maleimide-based porphyrin monomer;
[0067] C) asymmetric addition reaction of divinyl ether and hydrogen bromide to obtain an intermediate of formula (h), and then react with TBD or quaternize to obtain vinyl ether monomer;
[0068] ...
Embodiment 1
[0108]
[0109] Triphenylphosphine (1.35 g, 5.15 mmol) was dissolved in 20 ml of dehydrated THF solution, and the reaction system was set to -78°C using a dry ice / acetone bath. Add diisopropyl azodicarboxylate DIAD (1.01ml, 5.15mmol) dropwise for about 2 minutes, and the solution turns yellow after the dropwise addition. After stirring for 10 minutes, 6-chloro-1-hexanol (1.16g, 8.5mmol) was added dropwise, and after 5 minutes, maleimide (0.5g, 5.15mmol) was added, and after returning to room temperature, it was reacted for 10h, and the reaction system was dark gray. After rotary evaporation of the solvent, the crude product was separated by silica gel column chromatography (eluent n-hexane / ethyl acetate=2 / 1), and the product EL1 was a light yellow solid with a yield of 66%. 1 HNMR (300MHz, CDCl 3 )δ=6.72, 3.68, 3.37, 2.15~2.07, 1.75~1.71MS (ESI):[C10H14ClNO2], m / z=215.7[M+H] + (calcd. 215.7).
[0110] Add p-hydroxybenzaldehyde (13.2g, 108mmol), p-bromobenzaldehyde (59.7...
Embodiment 2
[0116]
[0117] Add p-hydroxybenzaldehyde (13.2g, 108mmol), 2,4-dichlorobenzaldehyde (56.8g, 324mmol) and 500mL propionic acid, heat up to 130°C, add pyrrole (30mL, 432mmol) dropwise, continue Raise the temperature to 160°C for reflux reaction for 2 hours, cool to room temperature after the reaction, add methanol and cool in the refrigerator overnight, filter the resulting product through silica gel column chromatography (CHCl 3 / CH 3 OH) Purify and collect the second color band to obtain the product EL6 with a yield of about 9%. 1 H NMR (300MHz, CDCl 3 )δ=8.89,8.13,7.92,7.10,-2.82MS (MALDI-ToF):[C44H24Br6N4O], m / z=837.4[M+H] + (calcd. 837.4).
[0118] Under nitrogen protection, EL1 (0.26g, 1.2mmol), EL6 (0.84g, 1mmol), anhydrous potassium carbonate (0.28g, 2mmol) and catalytic amount of potassium iodide (10mg) were dissolved in 50ml of dehydrated DMF, 100 Reaction at ℃ for 12h. After the reaction, the potassium salt was removed by filtration, and the DMF was rotary ev...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com