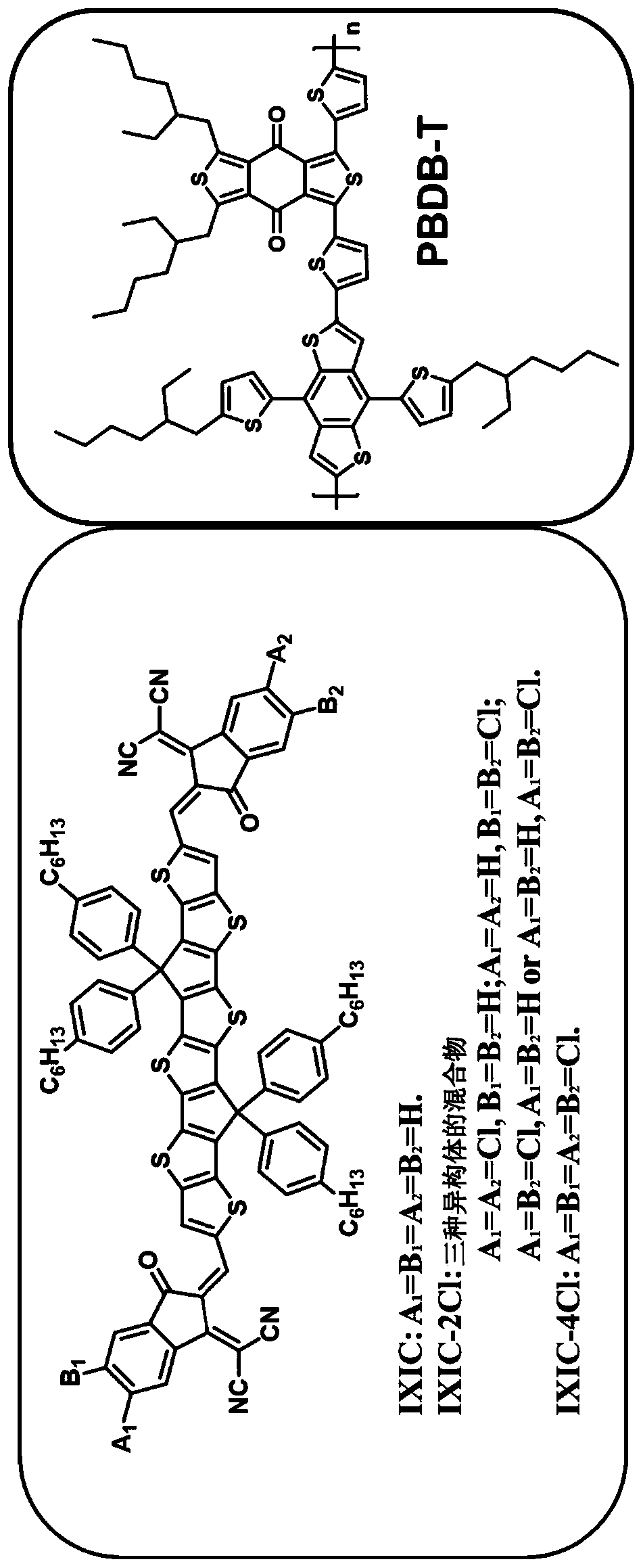
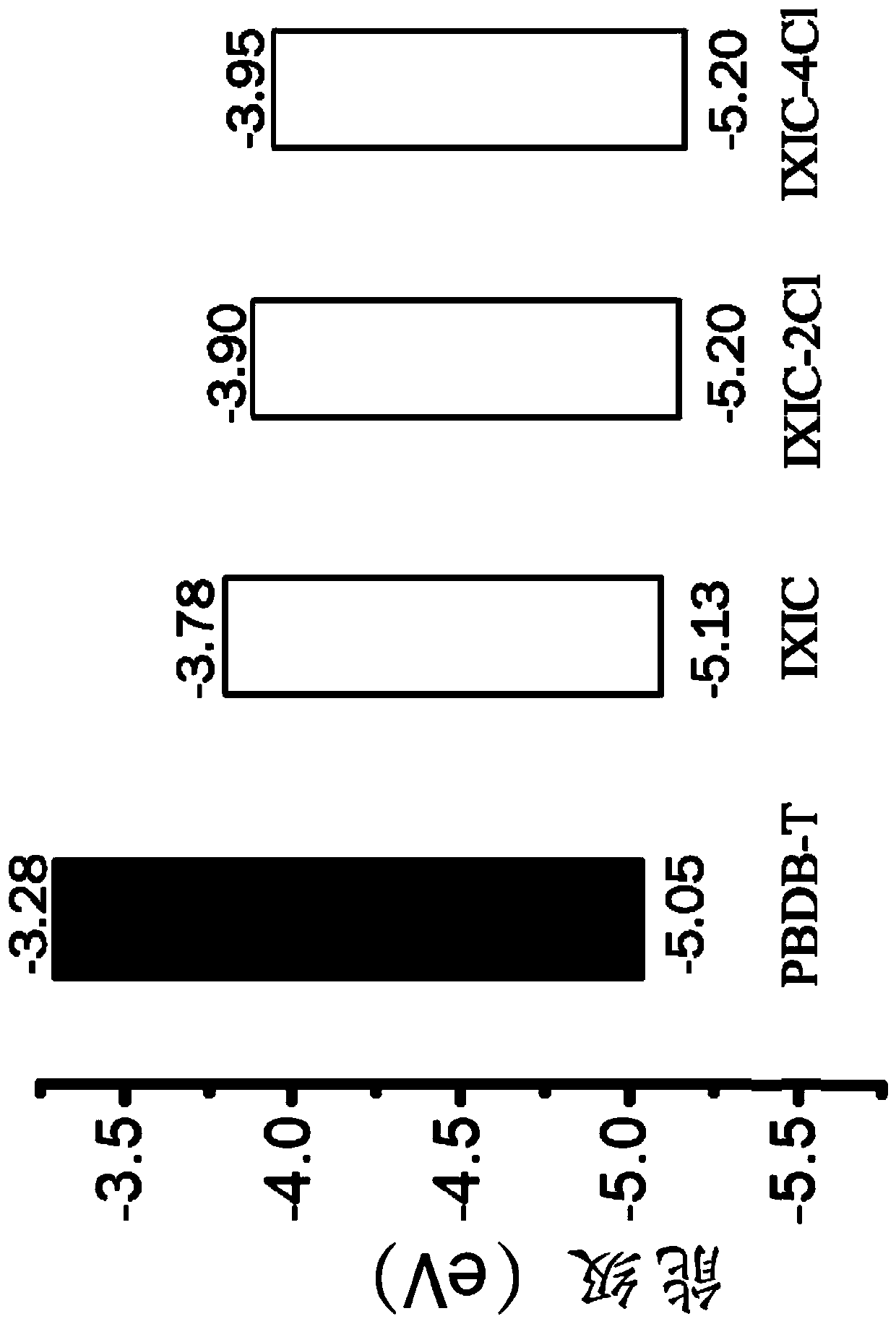
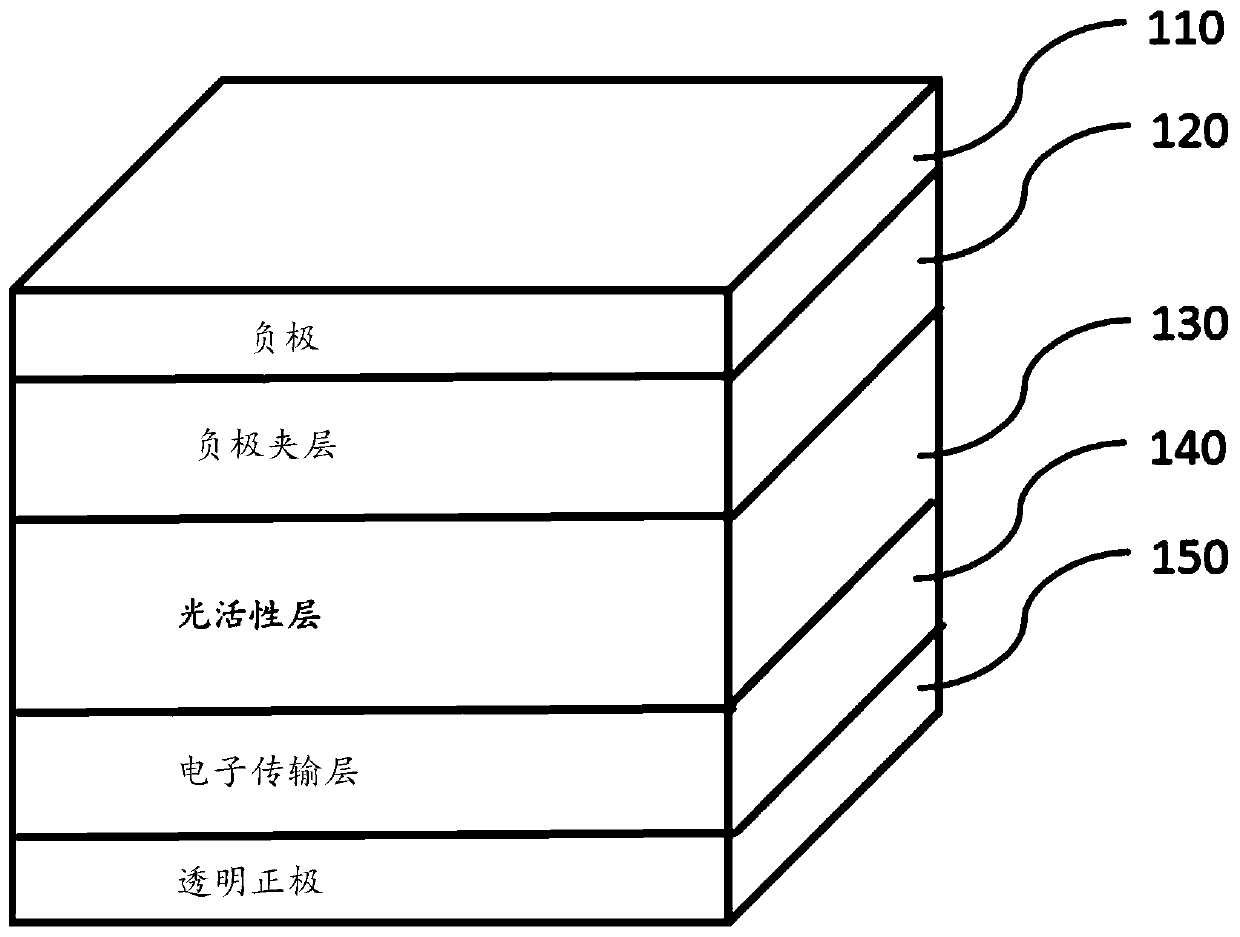
Thiophene-based fused aromatic systems
A technology of alkyl aryl and alkyl, applied in the field of preparation of photoactive layer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0245] The synthesis of embodiment 1-TTDTT-CHO
[0246]
[0247] At -78°C, to a solution of thiophene[3,2-b]thiophene (3.00 g, 21.40 mmol) in THF was added dropwise a 2.0 M solution of n-butyllithium in hexane (11.20 mL , 22.47mmol). The reaction mixture was stirred at -78 °C for 1 h, then TIPSCl (4.54 g, 23.53 mmol) was added. The mixture was returned to room temperature and stirred overnight. The reaction was quenched with water and extracted three times with ethyl acetate. The combined organic phases were washed with water and brine successively, and then the solution was washed with Na 2 SO 4 Dry and concentrate under reduced pressure. The crude product was purified by flash column chromatography (eluent: n-hexane) to give a white solid product (4.24 g, 67%).
[0248]
[0249] At -78°C, to compound 1 (4.24g, 14.28mmol) in THF solution, under N 2 A 2.0M solution of n-butyllithium in hexane (7.80 mL, 15.71 mmol) was added dropwise under protection. The reaction...
Embodiment 2-IX
[0258] The synthesis of embodiment 2-IXIC
[0259]
[0260] in N 2 , to TTTDT-CHO (60 mg, 0.053 mmol) and 1,1-dicyanomethylene-3-indanone (102 mg, 0.53 mmol) in anhydrous CHCl 3 To a solution in (10 mL) was added pyridine (0.1 mL). The mixture was refluxed for 16 h, then cooled to room temperature, and the mixture was poured into CH 3 OH (100mL) and filtered, the residue remaining in the filter paper was passed through CHCl 3 dissolve. After removal of the solvent, the residue was purified using silica gel column chromatography using petroleum ether / CH 2 Cl 2 (1:1, v / v) as eluent, a dark green solid (50 mg, 64%) was obtained. 1 H NMR (400MHz, CDCl 3 , ppm): δ=8.837(s, 2H), 8.664-8.644(m, 2H), 8.107(s, 2H), 7.912-7.891(m, 2H), 7.762-7.703(m, 4H), 7.212-7.155 (m, 16H), 2.581(t, 8H, J=7.8Hz), 1.604-1.526(m, 8H), 1.342-1.247(m, 24H), 0.862(t, 12H, J=6.8Hz); 13 CNMR (100MHz, CDCl 3 ,ppm):δ=188.502,160.525,152.739,149.361,148.531,147.357,143.165,142.844,140.264,139.752,...
Embodiment 3-IX
[0261] The synthesis of embodiment 3-IXIC-2F
[0262]
[0263] in N 2 , to TTDTT-CHO (54 mg, 0.047 mmol), 2-(5-fluoro-3-oxo-2,3-dihydro-1H-indan-1-alkylene)malononitrile and 2-( 6-Fluoro-3-oxo-2,3-dihydro-1H-indenyl-1-alkylene)malononitrile (100 mg, 0.47 mmol) in anhydrous CHCl 3 (10 mL) was added pyridine (0.1 mL). The mixture was refluxed for 16 h, then cooled to room temperature, and the mixture was poured into CH 3 OH (100mL) and filtered, the residue remaining in the filter paper was passed through CHCl 3 dissolve. After removing the solvent, use petroleum ether / CH 2 Cl 2 The residue was purified using column chromatography on silica gel (1:1, v / v) as eluent to give a dark green solid (41 mg, 57%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com