Benzothiadiazine methoxy acrylate derivatives and preparation method and application thereof
A technology of benzothiadiazine methoxy acrylic acid and derivatives, which is applied in the fields of botanical equipment and methods, chemicals for biological control, biocides, etc., can solve the problem of single action site, unreasonable use, etc problem, to achieve the effect of delaying the generation of resistance and novel structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
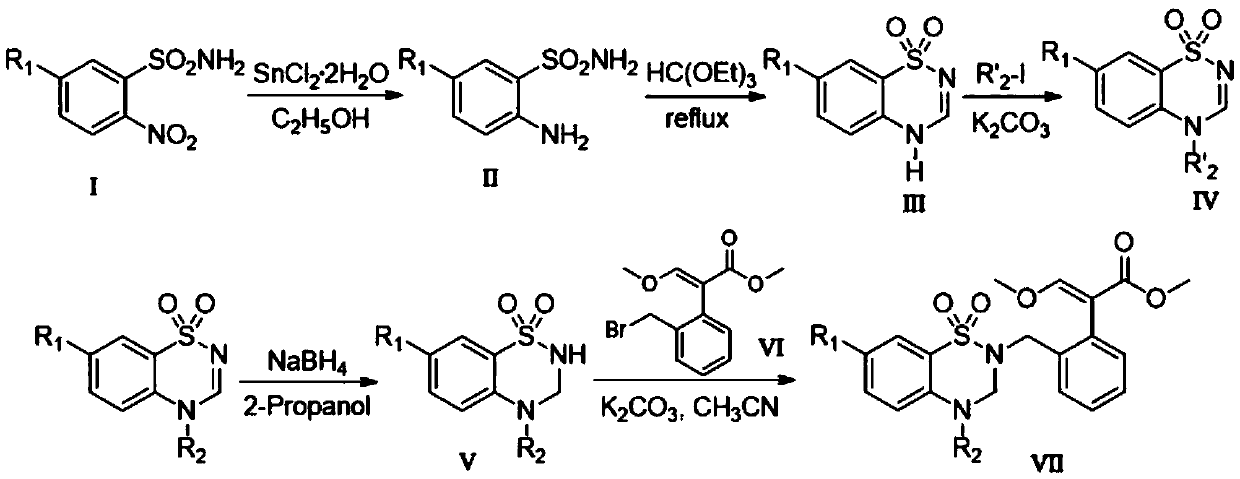
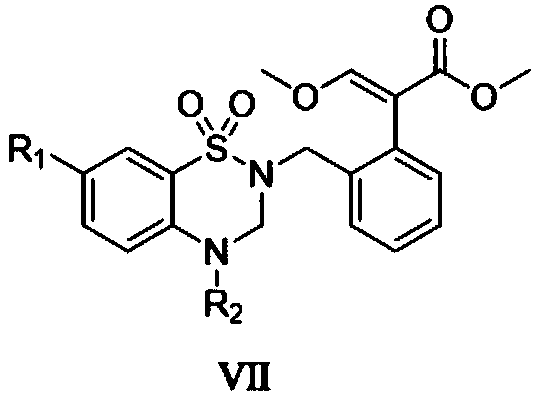
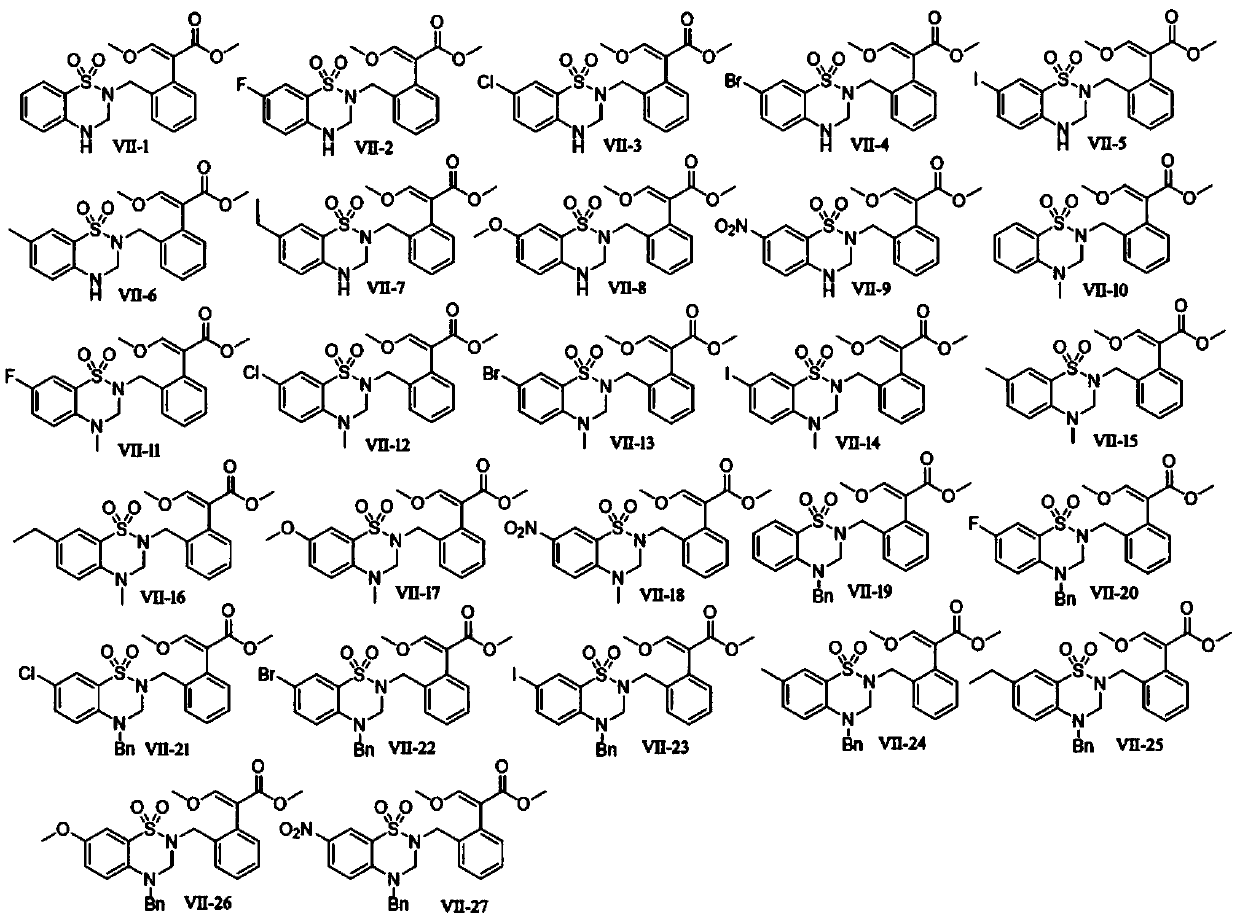
[0024] Step 1, prepare 2-aminobenzenesulfonamide
[0025] Add 4.04g (20.00mmol) of 2-nitrobenzenesulfonamide into a 150mL double-necked round-bottomed flask at room temperature, add 70mL of absolute ethanol and 6.78g (30mmol) of stannous chloride dihydrate in sequence, heat to reflux for 1h, and decompress In addition to absolute ethanol, 30 mL of ethyl acetate was added to the flask, and after stirring for 5 minutes, the mixture was poured into ice water, and solid sodium bicarbonate was added to adjust the pH to 8, filtered under reduced pressure, washed with ethyl acetate, and the filtrate was collected. Precipitation under reduced pressure gave 3.26 g of white solid 2-aminobenzenesulfonamide, with a yield of 95%.
[0026] Step 2. Preparation of 4H-benzo[e][1,2,4]thiadiazine 1,1-dioxide
[0027] Add 1.72g (10.00mmol) of 2-aminobenzenesulfonamide into a 50mL double-necked round-bottomed flask at room temperature, add 25mL of triethyl orthoformate, heat to reflux for 3h, low...
Embodiment 2
[0033] Step 1, preparation of 2-amino-5-fluorobenzenesulfonamide
[0034] Add 4.40g (20mmol) of 5-fluoro-2-nitrobenzenesulfonamide into a 150mL double-necked round bottom flask at room temperature, add 70mL of absolute ethanol and 6.78g (30mmol) of stannous chloride dihydrate in sequence, and heat to reflux for 1.2h , remove absolute ethanol under reduced pressure, add 30mL ethyl acetate to the flask, stir for 10min, pour the mixture into ice water, add solid sodium bicarbonate, adjust the pH to 8, filter under reduced pressure, wash with ethyl acetate, The filtrate was collected and precipitated under reduced pressure to obtain 3.65 g of white solid 2-amino-5-fluorobenzenesulfonamide with a yield of 96%.
[0035] Step 2. Preparation of 7-fluoro-4H-benzo[e][1,2,4]thiadiazine 1,1-dioxide
[0036]Add 1.9g (10mmol) 2-amino-5-fluorobenzenesulfonamide to a 50mL double-necked round bottom flask at room temperature, add 25mL triethyl orthoformate, heat to reflux for 2.5h, TLC monito...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com