Preparation method and application of tetra-amido macrocyclic ligand and metal chelate thereof
A metal chelate and macrocyclic ligand technology, applied in the restoration of polluted soil, organic chemistry, etc., can solve the problems of complex synthesis steps, environmental and human hazards, complex synthesis process, etc., and achieve easy control of synthesis conditions, The effect of improving economic efficiency and simple synthetic route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
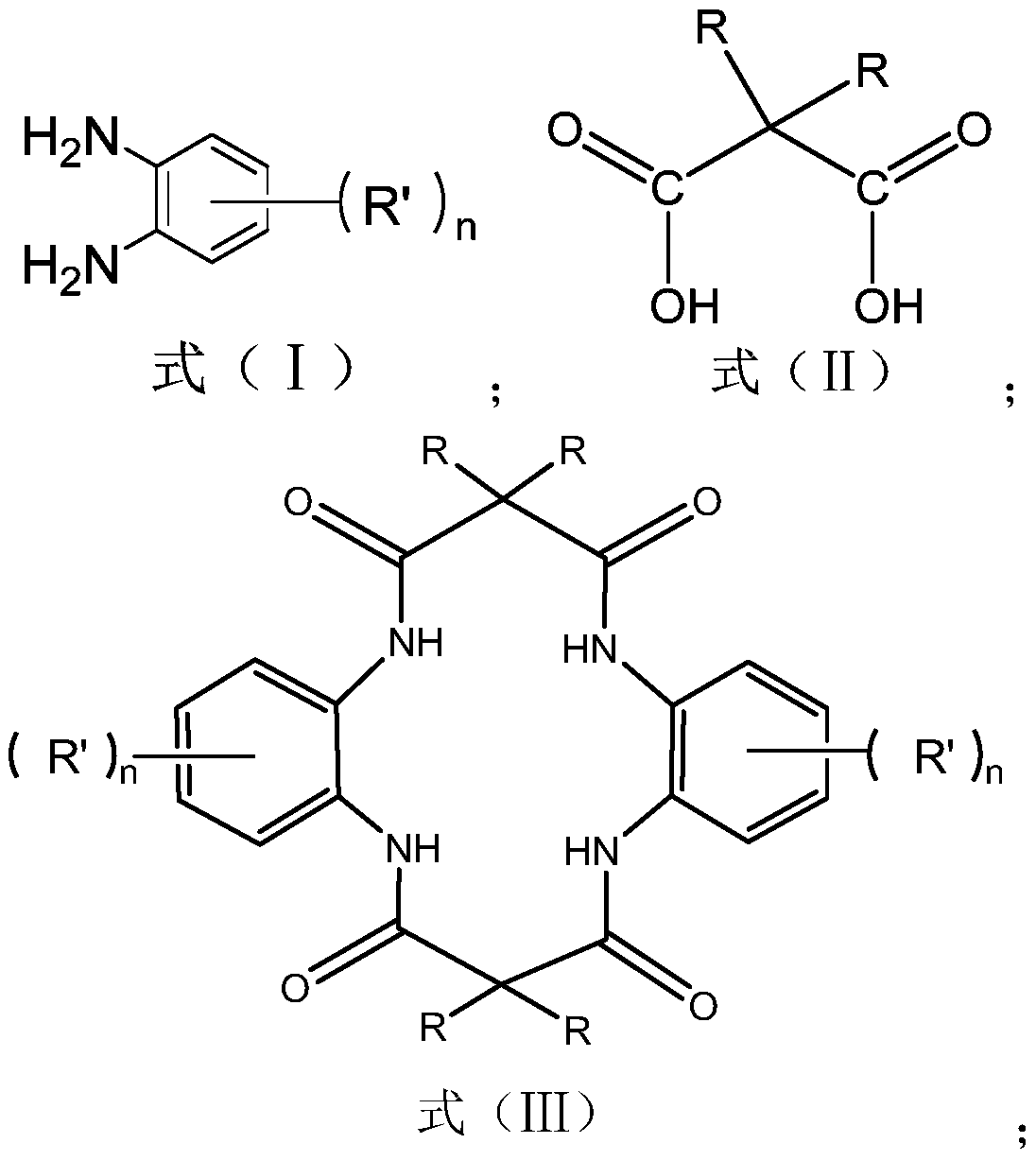
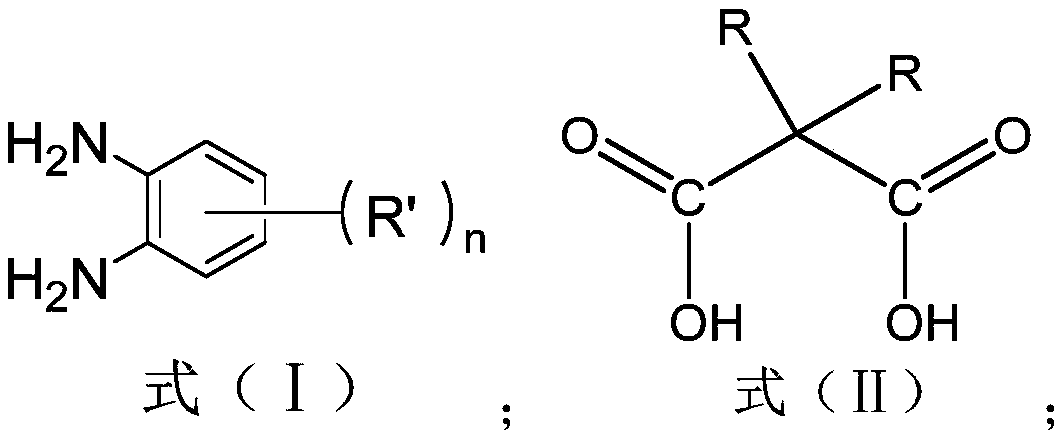
[0067] This embodiment provides a method for preparing a tetraamido macrocyclic ligand, comprising the following steps:
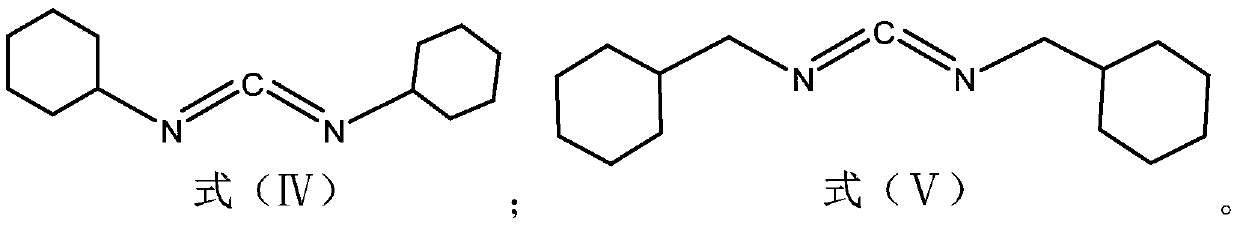
[0068] (1) In a 500mL round-bottomed flask, add 70mL of triethylamine into the ethanol solution, and use the ethanol solution to adjust the volume to 300mL; In the ethanol solution, heat at 60°C in a water bath, and use magnetic stirring under reflux at a speed of 800rpm to obtain a mixed solution A; in another 500mL round bottom flask, mix 52g dimethylmalonic acid with 211.5 g dicycloethylcarbodiimide was mixed in 300mL ethanol solution to obtain mixed solution B.
[0069] (2) Add the mixed solution B dropwise to the mixed solution A at a speed of 1 drop per second, and stir at a speed of 800rpm for 5h; After stirring, the solution was stirred for another 4h after the addition, and allowed to settle for 24h.
[0070] (3) Add deionized water to the above solution drop by drop until the top layer of the crystal is dissolved, vacuum filter the solution thro...
Embodiment 2
[0072] This embodiment provides a method for preparing a transition metal tetraamide-based macrocyclic ligand oxidation catalyst, comprising the following steps:
[0073] (1) Weigh 4.06 g of the tetraamido-macrocyclic ligand synthesized in Example 1 and put it in a 500 mL Schlenk bottle, add 160 mL of tetrahydrofuran distilled from water at the same time, stir at a speed of 500 rpm, and feed nitrogen As a protective gas, the flow rate is 100mL / min, then quickly add 10mL of n-butyllithium, keep stirring in the ice-water bath for 15min, then remove the ice-water bath, and stir at 25°C for 15min.
[0074] (2) Add 1.5g of anhydrous ferrous chloride to the above system, stir for 12h with a rotating speed of 500rpm, turn off the nitrogen protection, continue to stir for 3h in the air, filter to obtain a solid, wash 5 times with dichloromethane, and finally obtain the formula ( A) Iron tetraamido macrocyclic ligand oxidation catalyst shown.
[0075]
Embodiment 3
[0077] This example provides a method for preparing a transition metal tetraamide-based macrocyclic ligand oxidation catalyst, the difference between the preparation method and Example 2 is that 1.5g of anhydrous ferrous chloride is replaced by 1.67g of anhydrous cupric chloride , keeping other conditions unchanged.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com