Application of sulfonated BINAP and polyether functionalized ionic liquid integrated chiral catalyst in asymmetric hydrogenation reaction
A technology of chiral catalysts and ionic liquids, applied in the field of chemistry and chemical engineering, can solve the problems of reduced catalytic efficiency, non-conformity with green chemistry, waste of resources, etc., and achieve the effects of reduced negative effects, high stability, and low loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Asymmetric Hydrogenation Catalyzed by Catalyst 1a-1 / Methyl Acetoacetate / Methanol System
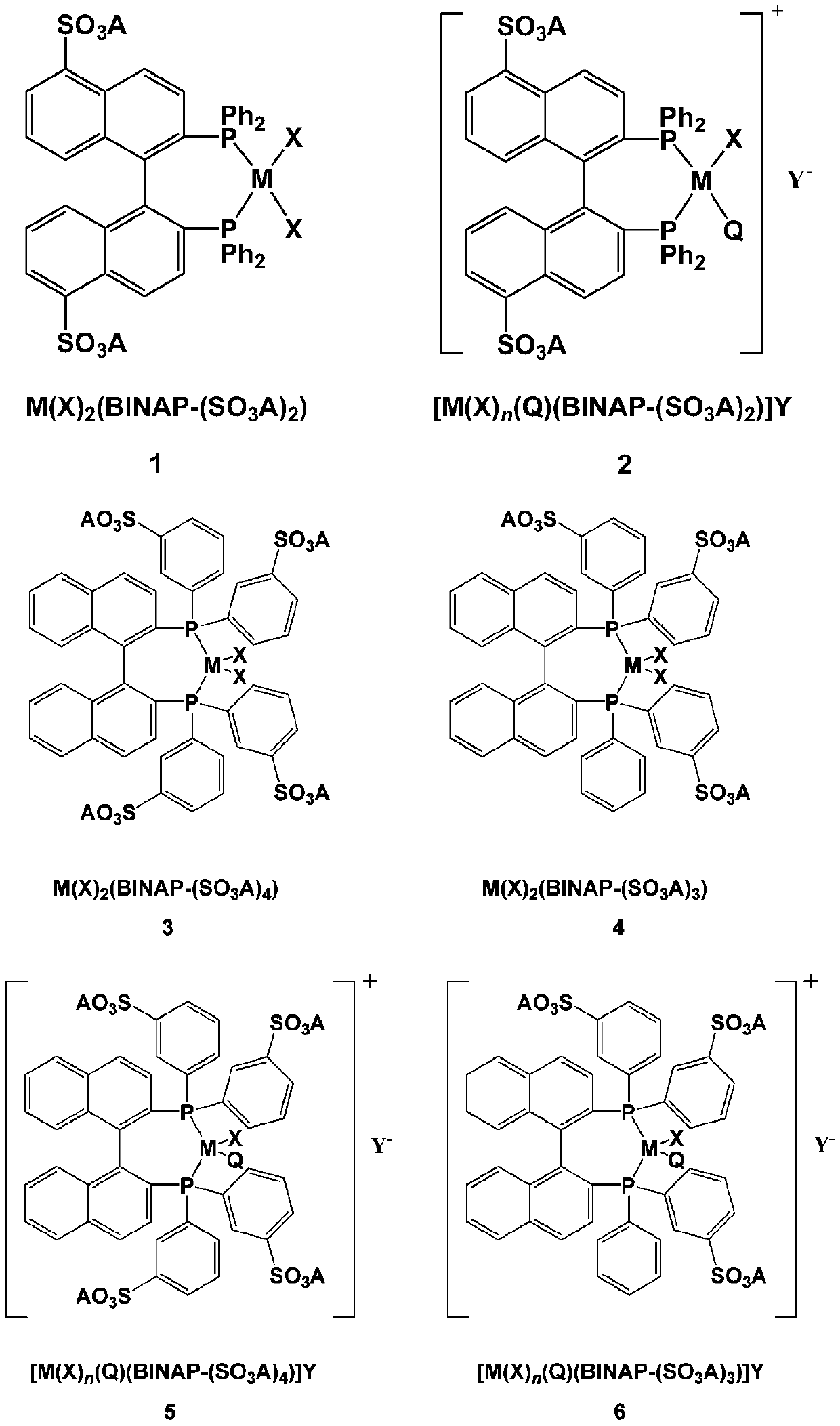
[0036] Catalyst 1a-1 is Ru(Br) 2 (S-BINAP-(SO 3 A) 2 )(A=[Ph(OCH 2 CH 2 ) 16 IMCH 3 ] + ,m=16,l=0,R 1 =Ph,R 2 = CH 3 ). Under an argon atmosphere, add catalyst 1a-1, methyl acetoacetate and methanol into the autoclave, the molar ratio of catalyst 1a-1 and methyl acetoacetate is 1:1000, then at 60°C, 4.0MPa hydrogen pressure The reaction was carried out for 20 hours. After the reaction, methanol was removed under reduced pressure, and n-hexane was added for extraction. The upper organic phase was analyzed by gas chromatography (lipodex A25m×0.25mm chiral capillary column), the conversion rate of the substrate was 100%, and the ee (S) value was 98.4 %; The initial TOF value of the reaction measured by the pressure drop method is 2914h -1 ; The catalyst phase of the lower floor continues to add methyl acetoacetate and methyl alcohol after the two-phase separation, and carr...
Embodiment 2
[0038] Asymmetric Hydrogenation Catalyzed by Catalyst 1a-2 / Methyl Acetoacetate / Methanol System
[0039] Catalyst 1a-2 is Ru(Br) 2 (S-BINAP-(SO 3 A) 2 )(A=[Ph(OCH 2 CH 2 ) 70 IMCH 3 ] + ,m=16,l=0,R 1 =Ph, R 2 = CH 3 ). Under argon atmosphere, add catalyst 1a-2, methyl acetoacetate and methanol into the autoclave, the molar ratio of catalyst 1a-2 and methyl acetoacetate is 1:1000, and then at 60°C, 4.0MPa hydrogen pressure The reaction was carried out for 20 hours. After the reaction, the methanol was removed under reduced pressure, and n-heptane was added for extraction. The upper organic phase was analyzed by gas chromatography (lipodex A25m×0.25mm chiral capillary column), the conversion rate of the substrate was 100%, and the ee (S) value 98.2%; The initial TOF value of the reaction measured by the pressure drop method is 2868h -1 ; The catalyst phase of the lower floor continues to add methyl acetoacetate and methyl alcohol after the two-phase separation, and ca...
Embodiment 3
[0041] Asymmetric Hydrogenation Catalyzed by Catalyst 1a-3 / Methyl Acetoacetate / Methanol System
[0042] Catalyst 1a-3 is Ru(Br) 2 (S-BINAP-(SO 3 A) 2 )(A=[n-C 12 h 25 (OCH 2 CH 2 ) 16 IMCH 3 ] + ,m=16,l=0,R 1 = n-C 12 h 25 , R 2 =CH 3 ). Under argon atmosphere, add catalyst 1a-3, methyl acetoacetate and methanol into the autoclave, the molar ratio of catalyst 1a-3 and methyl acetoacetate is 1:10000, and then at 80°C, 4.0MPa hydrogen pressure The reaction was carried out for 72 hours. After the reaction, methanol was removed under reduced pressure, and n-heptane was added for extraction. The upper organic phase was analyzed by gas chromatography (lipodex A 25m×0.25mm chiral capillary column), the substrate conversion rate was 95%, ee(S) Value 96.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com