Moxifloxacin hydrochloride sodium chloride injection and preparation method thereof
A technology of moxifloxacin hydrochloride and sodium chloride injection, which is applied in pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc., can solve the problem of uneven production and quality standards of activated carbon, concentration It is not easy to dissolve, and the activated carbon is flying, so as to ensure the effectiveness and safety, stable product quality, and reduce the pressure of environmental protection.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] The embodiment of the present invention provides a preparation method of moxifloxacin hydrochloride sodium chloride injection, comprising the following steps:
[0029] Add moxifloxacin hydrochloride, sodium chloride and pH regulator into the water for injection, control the temperature at 65-75°C, stir for 10-15min, after dissolving evenly, test according to the intermediate quality standard, test the content, pH value, visible foreign matter , after passing the test, filter through 3 or 4 groups of polyethersulfone filter elements with sequentially decreasing pore diameters, fill, sterilize, and pack after passing the light inspection to obtain the moxifloxacin hydrochloride sodium chloride injection product.
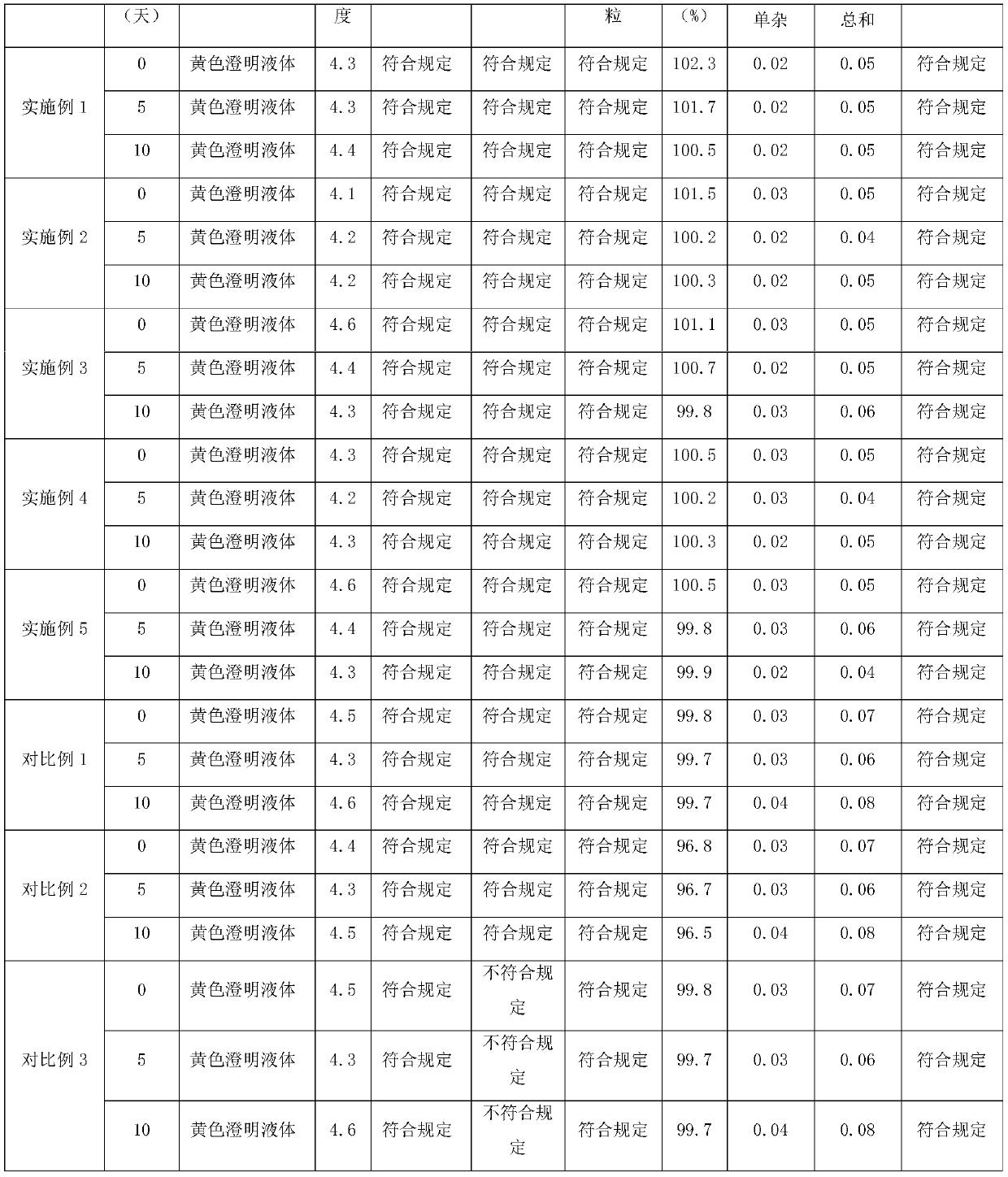
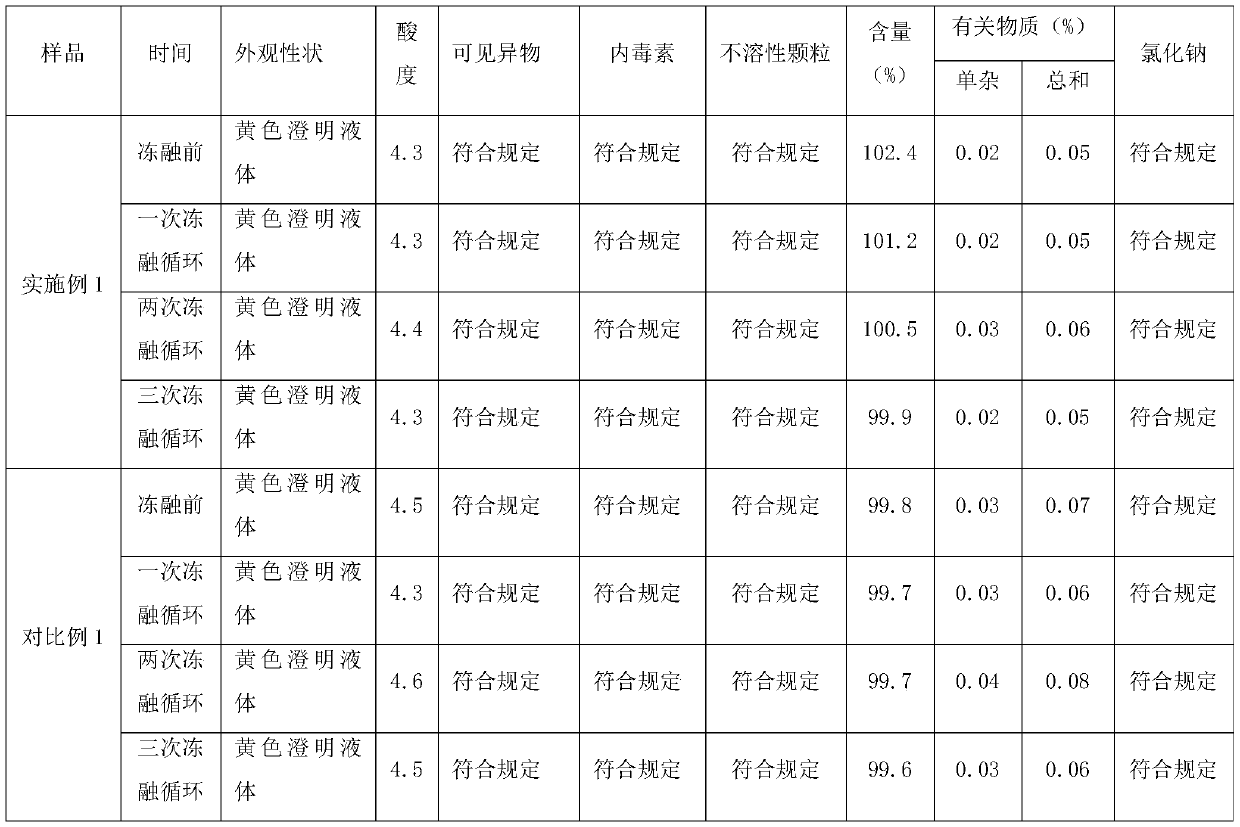
Embodiment 1
[0032] A preparation method of moxifloxacin hydrochloride sodium chloride injection, comprising the steps of:
[0033] Prescription: Moxifloxacin hydrochloride (calculated as moxifloxacin) 400mg, sodium chloride 2.0g, water for injection 250mL, pH adjuster (sodium hydroxide or hydrochloric acid) appropriate amount, filling specification 0.4g / 250mL, calculated in 1000 bags.
[0034] S1: Add water for injection with 50% of the total volume of the injection in the concentrated preparation tank, control the temperature at 65°C, add the prescribed amount of moxifloxacin hydrochloride and sodium chloride in sequence, stir to dissolve, and pass through the polyether with a pore size of 1.0 μm The sulfone filter element A is used for filtering, and the polyethersulfone filter element A is washed with water for injection with 15% of the total volume of the injection solution, and the flushing solution and the filtrate are sent to the dilute distribution tank together;
[0035] S2: Add ...
Embodiment 2
[0037] A preparation method of moxifloxacin hydrochloride sodium chloride injection, comprising the steps of:
[0038] Prescription: Moxifloxacin hydrochloride (calculated as moxifloxacin) 413mg, sodium chloride 2.1g, water for injection 255mL, appropriate amount of pH regulator (sodium hydroxide or hydrochloric acid), the filling specification is 0.4g / 250mL, calculated in 1000 bags.
[0039] S1: Add water for injection with 30% of the total volume of the injection in the concentrated preparation tank, control the temperature at 75°C, add the prescribed amount of moxifloxacin hydrochloride and sodium chloride in sequence, stir to dissolve, and pass through the polyether with a pore size of 1.0 μm The sulfone filter element A is used for filtering, and the polyethersulfone filter element A is washed with water for injection with 20% of the total volume of the injection solution, and the flushing solution and the filtrate are sent to the dilute distribution tank together;
[004...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com