Fusion protein, as well as efficient expression method and application thereof
A fusion protein and high-efficiency expression technology, applied in the field of fusion proteins, can solve problems such as difficult to achieve high-efficiency expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The construction, expression, in vitro enzymatic catalysis and detection of fusion protein gene recombinant Escherichia coli, the specific implementation steps are:
[0030] (1) AtSUS1 and RsUGT72B14 gene optimization: On the premise of not changing the amino acid sequence, the E. coli preferred codon optimized sequence was used.
[0031] The optimized AtSUS1 nucleotide sequence is shown in SEQ ID NO.2;
[0032] The optimized nucleotide sequence of RsUGT72B14 is shown in SEQ ID NO.3, and the GenBank number is KU523897.1.
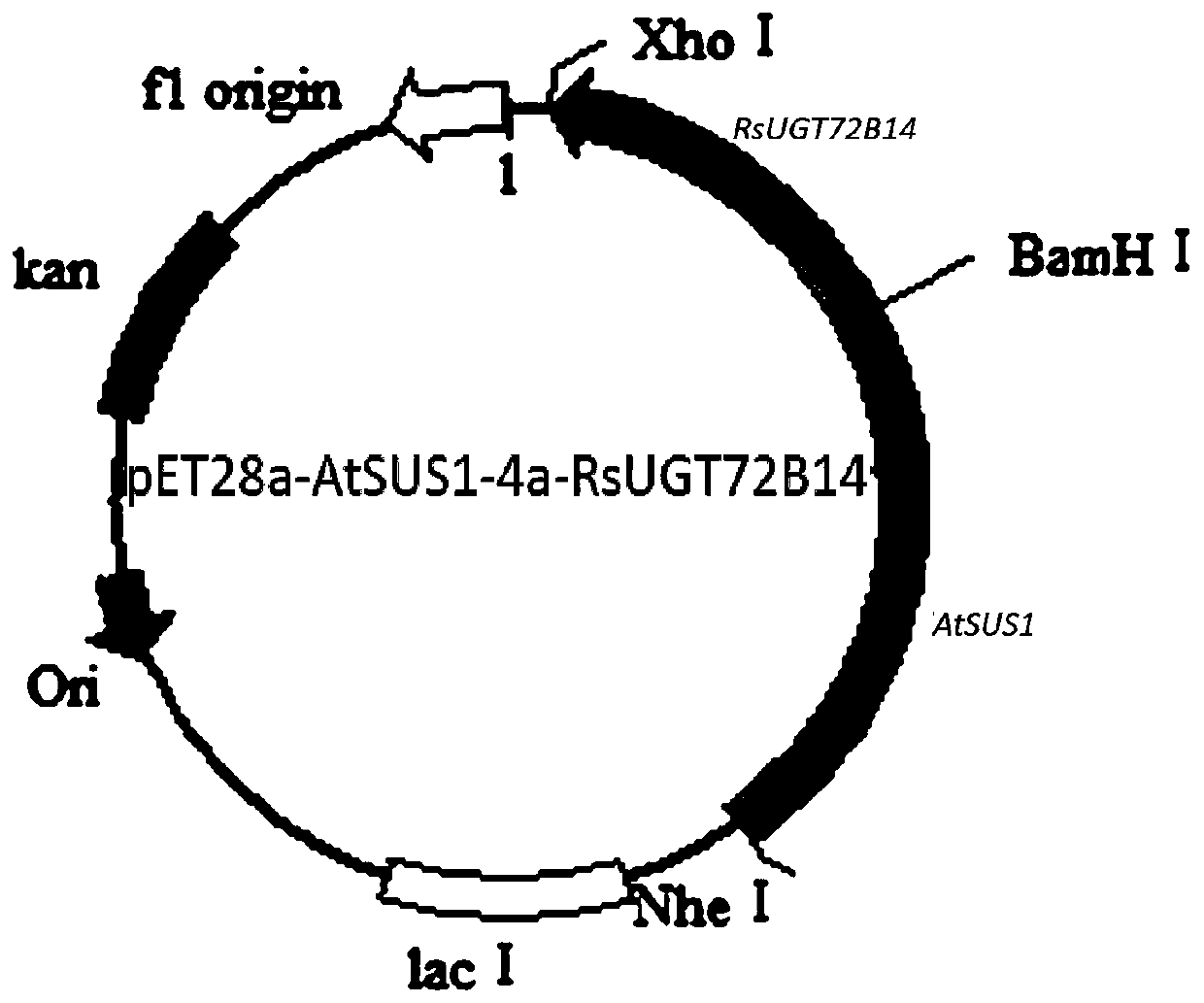
[0033] (2) Construction of AtSUS1-RsUGT72B14 fusion gene: Between AtSUS1 and RsUGT72B14 optimized gene sequences, a linker peptide sequence GGTGGATCCGGT including restriction site BamH I (GGATCC) and encoding four amino acids of GGSG was designed; SEQ ID NO.4. The AtSUS1-RsUGT72B14 fusion protein gene was synthesized according to the sequence of SEQ ID NO.1.
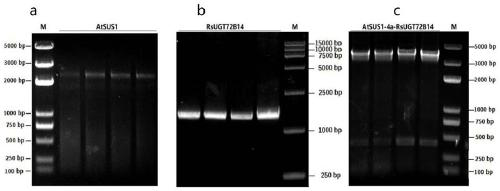
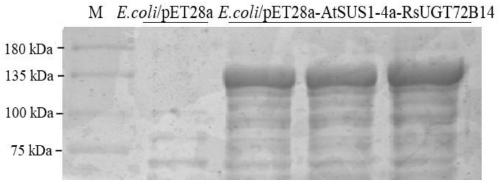
[0034] The AtSUS1 gene, RsUGT72B14 gene and AtSUS1-RsUGT72B14 fusion protein gene synthesi...
Embodiment 2
[0043] The construction, expression, and whole-cell catalysis and detection of fusion protein gene recombinant Escherichia coli, the specific implementation steps are:
[0044] Perform the same steps (1)-(3) in Example 1 to obtain recombinant Escherichia coli, then induce expression for 6 hours, adjust the temperature to 30°C for reaction, add 200 μM tyrosol, 200 μM sucrose, and 10 μM UDP, continue to cultivate for 8 to 12 hours, collect and culture After ultrasonic crushing of the liquid, freeze-drying treatment, the freeze-dried powder is extracted with an equal volume of methanol, and after the impurity is passed through the membrane, it is detected by HPLC with the step (6) of Example 1, and the result ~ 4min peak (such as Figure 4 c), indicating that the AtSUS1-RsUGT72B14 fusion protein can also catalyze the synthesis of salidroside in Escherichia coli cells. At the same time, under the same reaction conditions, the recombinant Escherichia coli containing only the RsUGT7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com