Detection method for thyroid cancer related gene fusion mutation and kit
A detection kit and gene fusion technology, applied in the field of genetic engineering, can solve the problems of long detection cycle, high cost, and low detection resolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
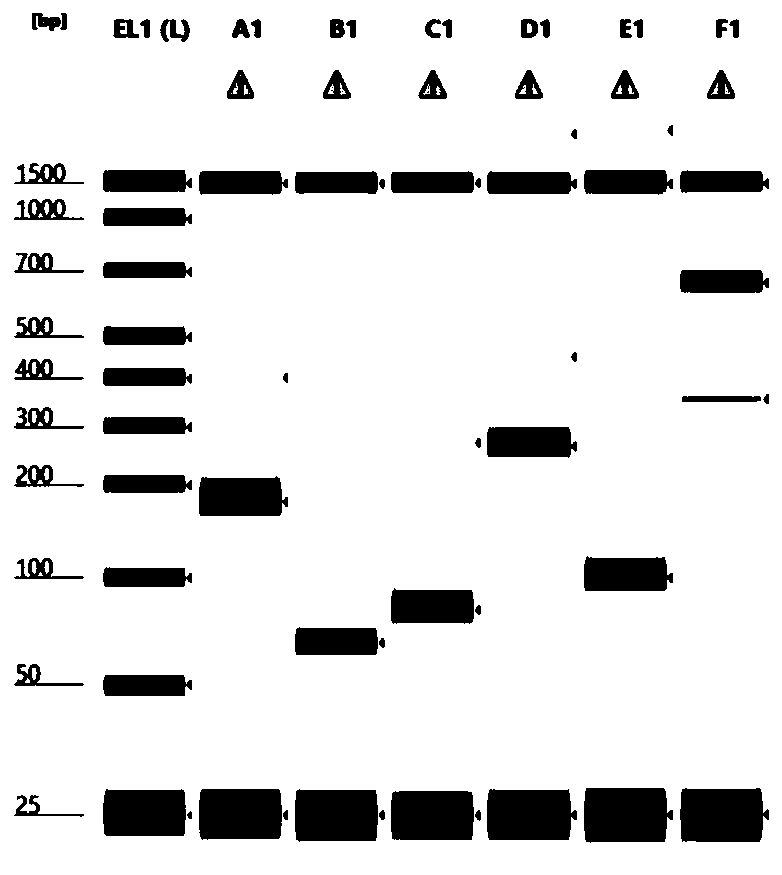
[0046] Embodiment 1 Detection kit for thyroid cancer-related gene fusion mutation based on the detection method for thyroid cancer-related gene fusion mutation provided by the present invention:
[0047] The detection kit includes the reagents required for RT-PCR detection, thyroid cancer-related fusion mutation gene specific primers, internal reference gene primers, negative RNA controls, and positive RNA controls.
[0048] The thyroid cancer-related gene fusion mutations include RET / PTC1 fusion, RET / PTC3 fusion and PAX8-PPARG rearrangement.
[0049] The reagents required for the above RT-PCR detection include SuperScript TM IV reverse transcriptase and 2×PlatinumSuperFi II PCR reaction mixture (product of Invitrogen company).
[0050] Thyroid cancer-related fusion mutant gene-specific primers and internal reference gene primers are the primers shown in SEQ ID NO. 1-12.
[0051] The above-mentioned negative RNA control substance is human total RNA that does not carry the a...
Embodiment 2
[0053] Embodiment 2 A detection method for thyroid cancer-related gene fusion mutation (using the detection kit described in Embodiment 1):
[0054] (1) Extract total RNA from the test sample;
[0055] The test sample was a paraffin-embedded sample (product number: 0710-0496) containing the three gene fusion mutations purchased from SeraCare Company in the United States. Using Quick-DNA / RNA from Zymo Research, USA TM FFPE Kits (Product No.: R1009) were used to extract total RNA from paraffin samples. The extraction method was carried out according to the instructions of the kit.
[0056] The extracted RNA was FFPE sample RNA containing RET / PTC1, RET / PTC3, PAX8 / PPARg.
[0057] At the same time, a negative control group was set up, and a negative control RNA sample was taken, which was purchased from Thermo Fisher (product number 361787), which did not contain any of RET / PTC1, RET / PTC3, and PAX8 / PPARg.
[0058] (2) The above-mentioned FFPE samples and negative control RNA s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com