Imidapril hydrochloride tablet and preparation method thereof
A technology of imidapril hydrochloride tablet and imidapril hydrochloride, which is applied in the directions of pill delivery, pharmaceutical formulation, and medical preparations of inactive ingredients, etc., can solve problems such as uneven content distribution, achieve stable dissolution effect, The effect of improving drug content uniformity and overcoming uneven content distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1, the preparation of imidapril hydrochloride tablet
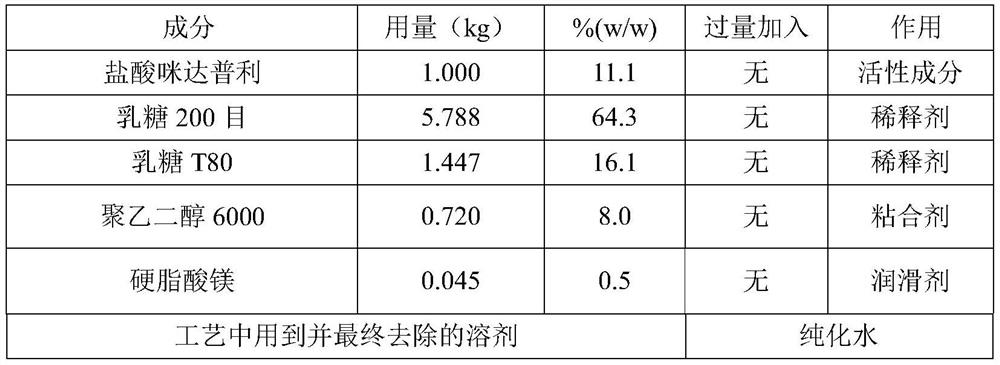
[0044] The prescription of imidapril hydrochloride tablet of 10mg specification is as shown in Table 1 (batch 100,000 tablets).
[0045] Table 1
[0046]
[0047] Purified water is used in the formulation of the binder and is removed in the drying of the granules.
[0048] The preparation method of imidapril hydrochloride tablet comprises the steps:
[0049] 1. Pretreatment of raw and auxiliary materials: Take imidapril hydrochloride as raw material and control the particle size D 90 <30μm; Magnesium stearate passed through 80 mesh, set aside.
[0050] 2. Take the polyethylene glycol of the prescribed amount, slowly add it into the constantly stirring water until it is completely dissolved, and prepare it into an aqueous solution with a concentration of 50% (w / w).
[0051] 3. First mix the 200-mesh lactose and lactose T80 According to the mass ratio of 4:1, the mixed lactose is obtained evenly. T...
Embodiment 2
[0058] Embodiment 2, the preparation of imidapril hydrochloride tablet
[0059] The prescription of imidapril hydrochloride tablet of 10mg specification is as shown in Table 1 (batch 100,000 tablets).
[0060] Table 2
[0061]
[0062] Purified water is used in the formulation of the binder and is removed in the drying of the granules.
[0063] The preparation method of imidapril hydrochloride tablet comprises the steps:
[0064] 1. Pretreatment of raw and auxiliary materials: Take imidapril hydrochloride as raw material and control the particle size D 90 <30μm; Magnesium stearate passed through 80 mesh, set aside.
[0065] 2. Take the prescribed amount of polyethylene glycol, slowly add it into the constantly stirring water until it is completely dissolved, and prepare an aqueous solution with a concentration of 50% (w / w).
[0066] 3. First mix the 200-mesh lactose and lactose T80 According to the mass ratio of 4:2, the mixed lactose is obtained evenly. Then weigh a...
Embodiment 3
[0073] Embodiment 3, the preparation of imidapril hydrochloride tablet
[0074] The prescription of imidapril hydrochloride tablet of 10mg specification is as shown in Table 1 (batch 100,000 tablets).
[0075] table 3
[0076]
[0077]
[0078] Purified water is used in the formulation of the binder and is removed in the drying of the granules.
[0079] The preparation method of imidapril hydrochloride tablet comprises the steps:
[0080] 1. Pretreatment of raw and auxiliary materials: Take imidapril hydrochloride as raw material and control the particle size D 90 <30μm; Magnesium stearate passed through 80 mesh, set aside.
[0081] 2. Take the prescribed amount of polyethylene glycol, slowly add it into the constantly stirring water until it is completely dissolved, and prepare it into an aqueous solution with a concentration of 45% (w / w).
[0082] 3. First mix the 200-mesh lactose and lactose T80 According to the mass ratio of 4:1.5, the mixed lactose is obtained...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| friability | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com