Hypoxia sensitive nitrobenzene chitosan and its preparation and application
A nitrophenylated chitosan, sensitive technology, applied in the direction of organic active ingredients, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the difficulty and rapidity of chitosan glycolipid grafts drug release and other issues to achieve the effect of improving drug efficacy and improving release efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
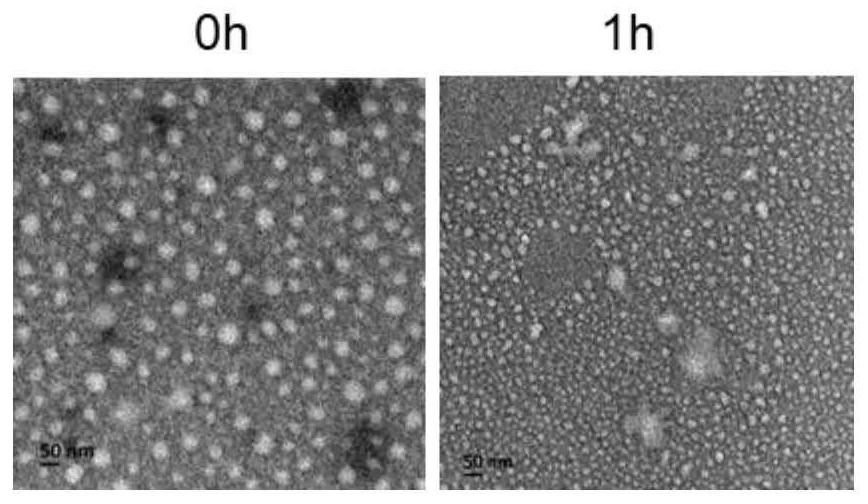
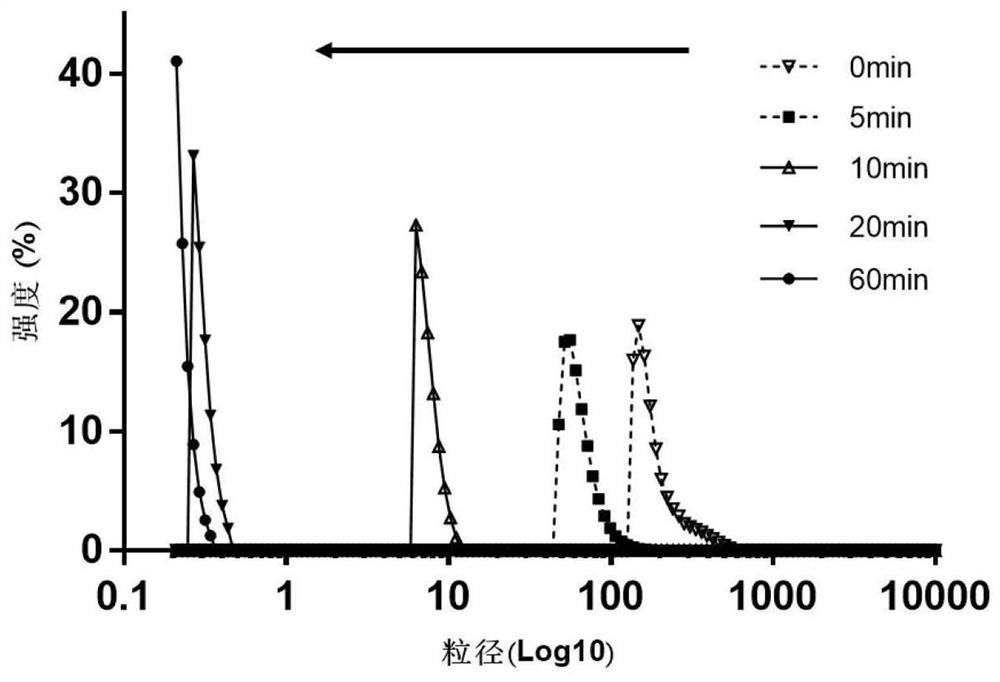
Examples
Embodiment 1
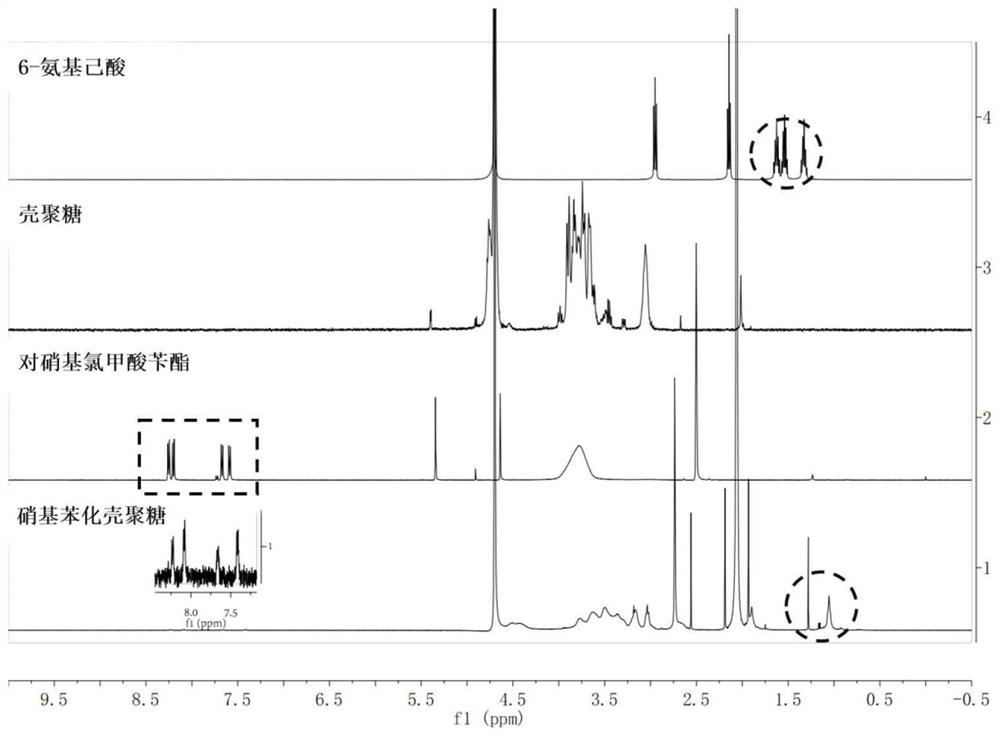
[0026] 1. Synthesis of Nitrophenylated Chitosan
[0027] Accurately weigh 210.1mg benzyl p-nitrochloroformate, 106.2mg 6-aminocaproic acid and 542μL triethylamine, suspend in 15mL dichloromethane, place in a 50mL dry round bottom flask, nitrogen atmosphere, ice-water bath for 10min, room temperature Reaction 12h. After the reaction, extract with 75mL deionized water and 15mL ethyl acetate, shake at room temperature with a vortex shaker, and let it stand still. After layering, take the upper layer of ethyl acetate, put it in a dry round bottom flask, and remove the solvent by rotary evaporation at 40°C. Add 15mL of N,N-dimethylformamide, 747.2mg of carbodiimide, 1034.7mg of chitosan (Mw=5000Da) and 15mL of deionized water into the flask, and heat at a constant temperature in a magnetic stirrer at 50°C for 16h to obtain the reaction product. The reactant was dialyzed in a dialysis bag (MWCO 3500Da) for 3 days to remove N,N-dimethylformamide and water-soluble by-products. Afte...
Embodiment 2
[0035] 1. Synthesis of Nitrophenylated Chitosan
[0036] Accurately weigh 224.1mg of benzyl p-nitrochloroformate, 272.7mg of 6-aminocaproic acid and 437μL of triethylamine, suspend in 10mL of dichloromethane, place in a 50mL dry round bottom flask, nitrogen atmosphere, ice-water bath for 10min, room temperature Reaction 24h. After the reaction, add 50 mL of deionized water and 10 mL of ethyl acetate for extraction, shake at room temperature with a vortex oscillator, and let stand. After layering, take the upper layer of ethyl acetate, put it in a dry round bottom flask, and remove the solvent by rotary evaporation at 40 °C. Add 10mL of N,N-dimethylformamide, 1196.3mg of carbodiimide, 1164.2mg of chitosan (Mw=5000Da) and 10mL of deionized water into the flask, and heat the reaction in a magnetic stirrer at 60°C for 12h to obtain the reaction product. The reactant was dialyzed in a dialysis bag (MWCO 3500Da) for 2 days to remove N,N-dimethylformamide and water-soluble by-produ...
Embodiment 3
[0042] 1. Synthesis of Nitrophenylated Chitosan
[0043] Accurately weigh 258.6mg of benzyl p-nitrochloroformate, 314.7mg of 6-aminocaproic acid and 504μL of triethylamine, suspend in 10mL of dichloromethane, place in a 50mL dry round bottom flask, nitrogen atmosphere, ice-water bath for 10min, room temperature Reaction 24h. After the reaction, add 50 mL of deionized water and 10 mL of ethyl acetate for extraction, shake at room temperature with a vortex oscillator, and let stand. After layering, take the upper layer of ethyl acetate, put it in a dry round bottom flask, and remove the solvent by rotary evaporation at 40 °C. Add 10mL of N,N-dimethylformamide, 1380.2mg of carbodiimide, 447.8mg of chitosan (Mw=5000Da) and 10mL of deionized water into the flask, and heat at a constant temperature in a magnetic stirrer at 60°C for 12h to obtain the reaction product. The reactant was dialyzed in a dialysis bag (MWCO 3500Da) for 2 days to remove N,N-dimethylformamide and water-solu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com