Calcium-containing barium scandium aluminate for impregnated diffusion cathode and preparation method thereof
A barium scandium aluminate, diffusion cathode technology, applied in chemical instruments and methods, parts of discharge tubes/lamps, inorganic chemistry, etc., can solve the problems of narrow temperature range, low diffusion rate, uneven emission, etc. Consistent phase structure, lower immersion temperature, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
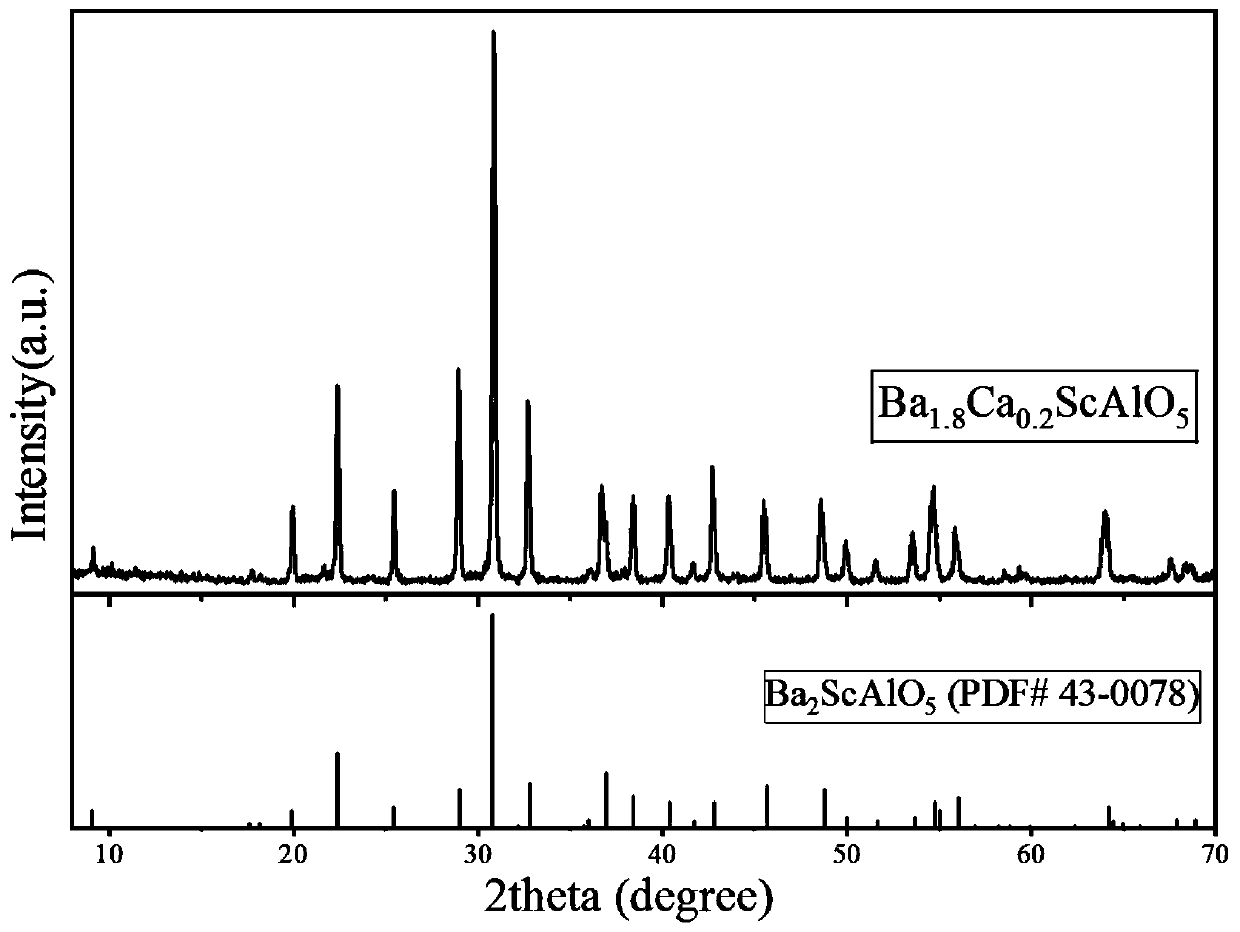
[0031] What this example prepares is Ba 1.8 Ca 0.2 ScAlO 5 Powder, prepared as follows:
[0032] According to Ba 2+ , Ca 2+ 、Sc 3+ 、Al 3+ The molar ratio of cations is 1.8:0.2:1:1 ratio to calculate 0.018mol of Ba(NO 3 ) 2 , 0.002mol of Ca(NO 3 ) 2 4H 2 O, 0.01mol of Sc(NO 3 ) 3 ·6H 2 O and 0.01mol of Al(NO 3 ) 3 9H 2O mass and accurately weighed. Put all the raw materials into a beaker, add 400 mL of deionized water to obtain a mixed solution with a total molar concentration of metal cations of 0.1 mol / L. The total number of moles of all metal cations in the mixed solution is 0.04mol. In order to completely precipitate the cations, the moles of the ammonium carbonate precipitation agent are weighed to be twice the sum of the moles of all cations, which is 0.08mol. After accurate weighing, it is configured into a 2.0mol / L sodium carbonate solution. Use a magnetic heating stirrer to make the mixed solution of the raw materials fully uniform, then pour the amm...
Embodiment 2
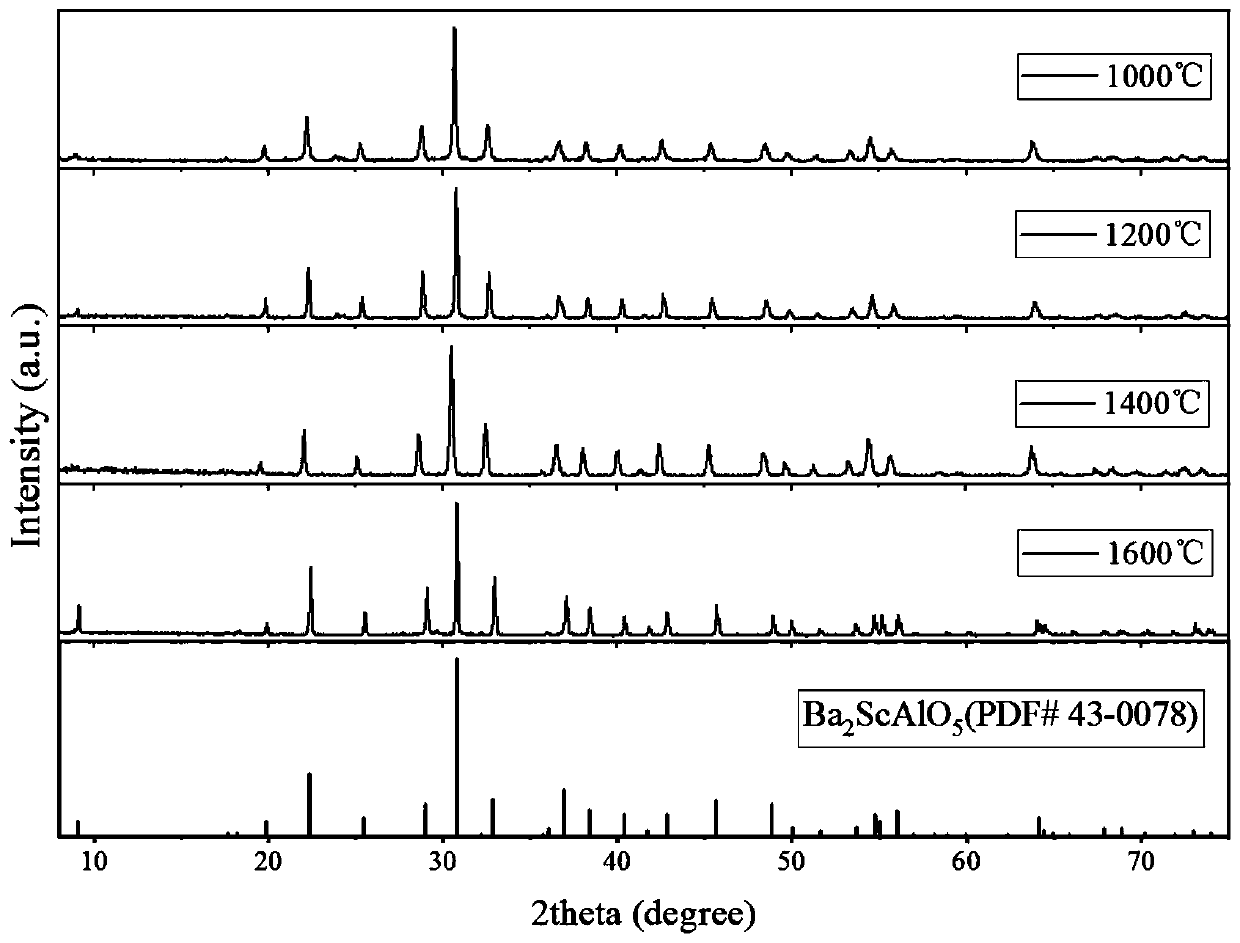
[0035] In order to study Ba 1.8 Ca 0.2 ScAlO 5 The temperature range where the phase structure can exist stably, because Ba 1.8 Ca 0.2 ScAlO 5 with Ba 2 ScAlO 5 The crystal structure of is similar, this embodiment has prepared the Ba of different temperature sintering 2 ScAlO 5 powder. The difference between this embodiment and embodiment 1 is that according to Ba 2+ 、Sc 3+ 、Al 3+ The molar ratio of cations is 2:1:1, and 0.02mol of Ba(NO 3 ) 2 , 0.01mol of Sc(NO 3 ) 3 ·6H 2 O and 0.01mol of Al(NO 3 ) 3 9H 2 The mass of O was accurately weighed and Ca was not added 2+ , the sintering temperature was adjusted to 1000°C, 1200°C, 1400°C and 1600°C respectively, and the other steps remained unchanged. The purpose is to prepare Ba at different sintering temperatures 2 ScAlO 5 , verify that the Ba produced by the present invention 2 ScAlO 5 It has a wide temperature range in which the phase structure can exist stably.
[0036] image 3 It is the Ba of differ...
Embodiment 3
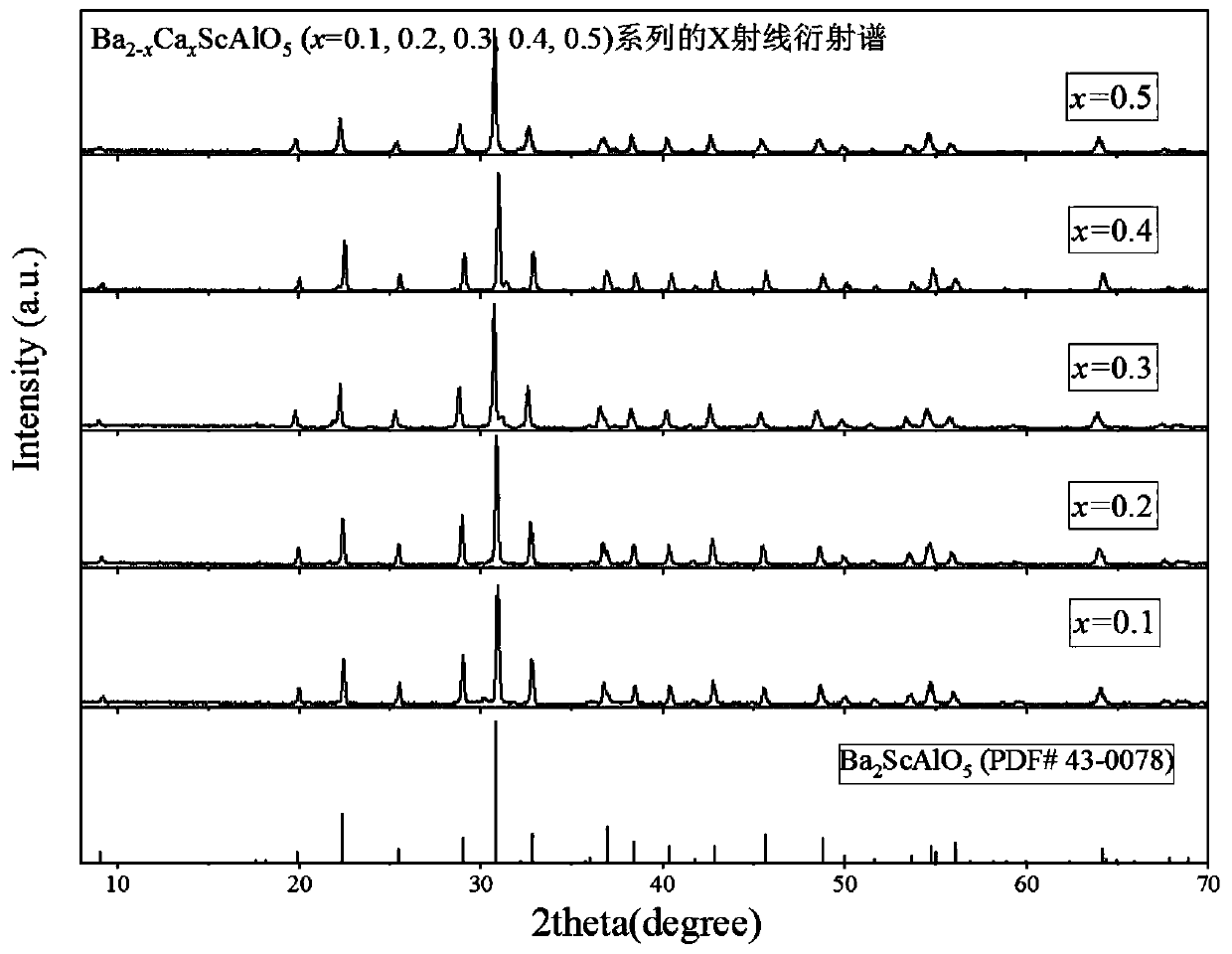
[0038] What this example prepares is Ba 1.5 Ca 0.5 ScAlO 5 Powder, the difference between this embodiment and embodiment 1 is that according to Ba 2+ , Ca 2+ 、Sc 3+ 、Al 3+ The molar ratio of cations is 1.5:0.5:1:1, and 0.015mol of Ba(NO 3 ) 2 , 0.005mol of Ca(NO 3 ) 2 4H 2 O, 0.01mol of Sc(NO 3 ) 3 ·6H 2 O and 0.01mol of Al(NO 3 ) 3 9H 2 The quality of O, and accurately weighed, other steps remain unchanged. The purpose is to adjust the ratio of each component to verify the importance of the raw material composition of the present invention.
[0039] Figure 4 Be that embodiment 3 makes Ba 1.5 Ca 0.5 ScAlO 5 Powder X-ray diffraction results, X-ray diffraction patterns and Ba 2 ScAlO 5 (β phase, PDF card number is 43-0078) The diffraction spectrum is consistent, indicating that the single-phase Ba was successfully prepared 2 ScAlO 5 .
[0040] The Ba that embodiment 3 obtains 1.5 Ca 0.5 ScAlO 5 The powder was used as an impregnating substance to prep...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com