Detection method of nadroparin calcium disaccharide spectrum
A technique for detecting nadroparin calcium and its detection method, which is applied in the field of raw drug detection, can solve the problems of inability to obtain the detection result of nadroparin calcium, low sensitivity, and high operation requirements, and achieve low performance requirements, high sensitivity, and detection low limit effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] This embodiment provides a method for detecting nadroparin calcium, comprising:
[0055] S1, the preparation of the solution:
[0056] (1) Preparation of enzymolysis buffer: Weigh 10 mg of bovine serum albumin and 32 mg of calcium acetate and dissolve them in 60 ml of water, then add 580 μl of glacial acetic acid to the solution, mix well and adjust the pH to 7.0 with 2M sodium oxide solution. Then make up to 100ml with water. Pass the prepared enzymatic hydrolysis buffer through a 0.22 μm microporous membrane for later use.
[0057] (2) Preparation of heparinase solution: mix equal amounts of heparinase I, heparinase II, and heparinase Ш, dilute to 0.4 IU / ml with enzymatic hydrolysis buffer, and set aside;
[0058] (3) Preparation of Nadroparin Calcium sample solution: take Nadroparin Calcium sample (label: 20181127), dilute with water and prepare a solution with a concentration of 20 μg / μl;
[0059] S2, complete enzymatic hydrolysis:
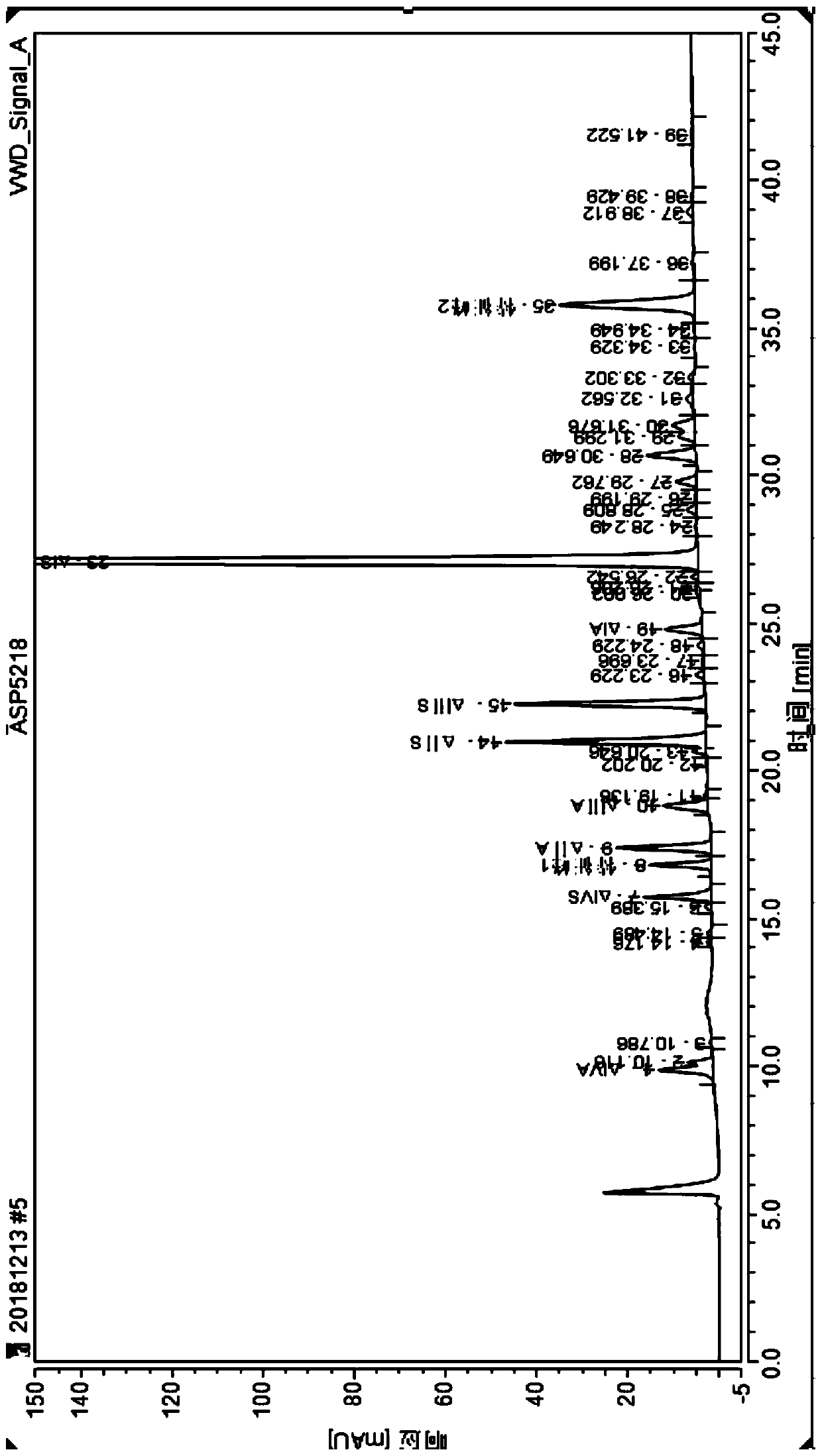
[0060] Take 10 μl of nadropa...
Embodiment 2
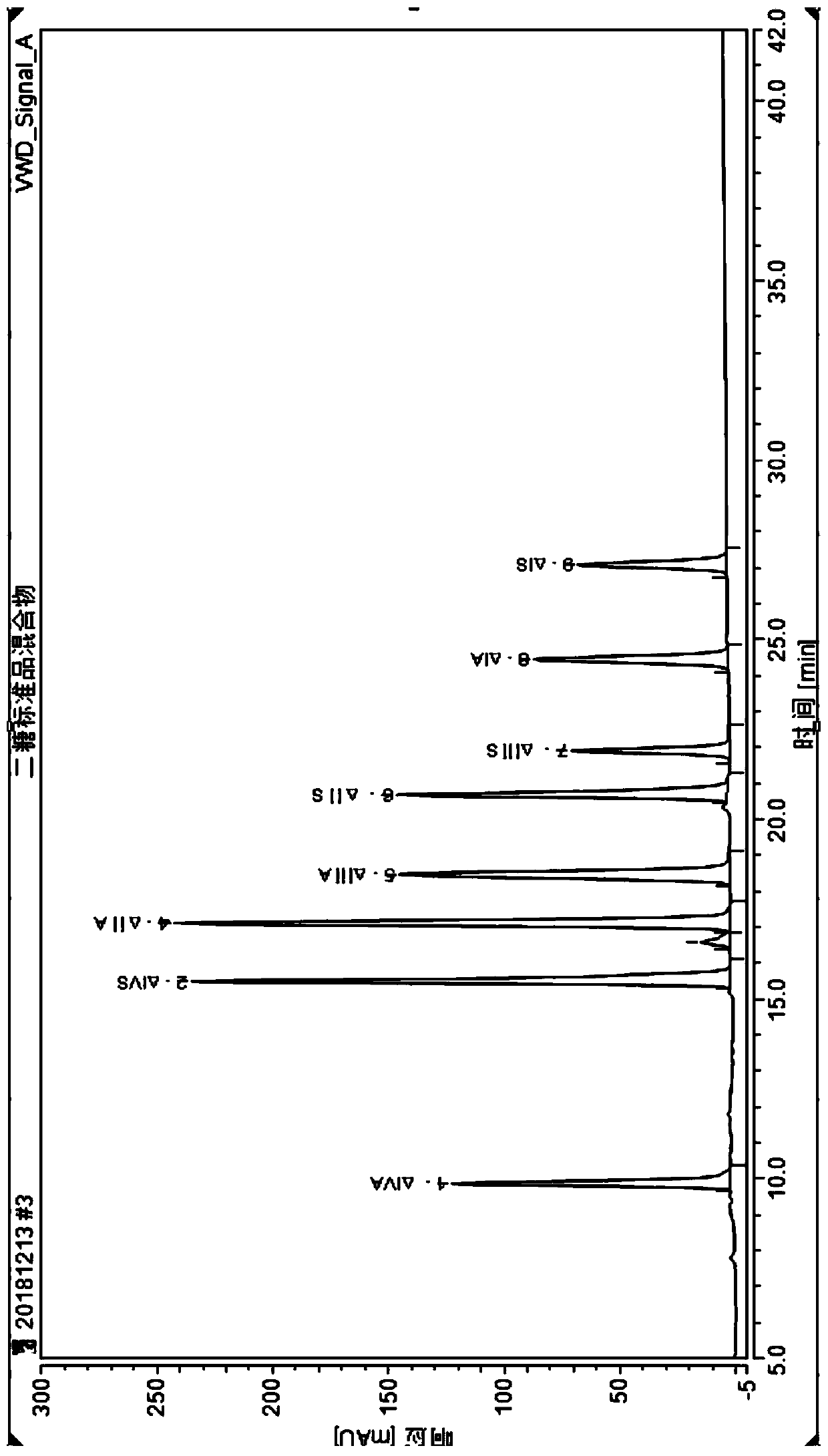
[0081] According to the method for detecting nadroparin calcium in Example 1, the difference is that: the nadroparin calcium sample is replaced by a nadroparin calcium intermediate sample (label: intermediate 1129-3). Chromatographic results such as Figure 5 .
[0082] Investigation test on the influence of enzymatic hydrolysis conditions on the test results:
[0083] 1. Condition investigation and verification of complete enzymatic hydrolysis:
[0084] According to the method for detecting nadroparin calcium in Example 1, the difference is: adjust the amount of heparanase added (20 μl, 40 μl, 60 μl, 80 μl, 100 μl, 120 μl) and adjust the enzymatic hydrolysis time (36h, 48h, 60h, 72h, 84h ).
[0085] The specific adjustment method is shown in Table 1.
[0086] Table 1
[0087] time Enzyme dosage 36h 20μl, 40μl, 60μl, 80μl, 100μl, 120μl 48h 20μl, 40μl, 60μl, 80μl, 100μl, 120μl 60h 20μl, 40μl, 60μl, 80μl, 100μl, 120μl 72h 20μl, 40μl, 60μl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com