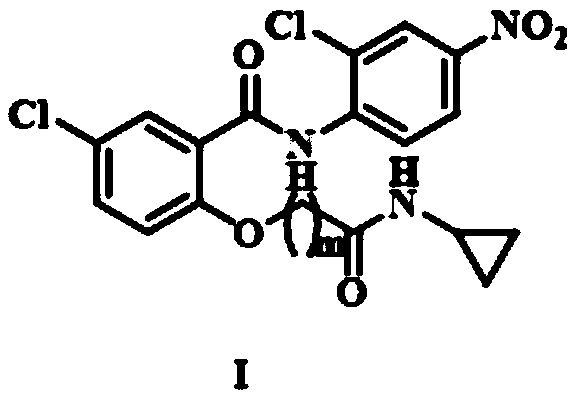
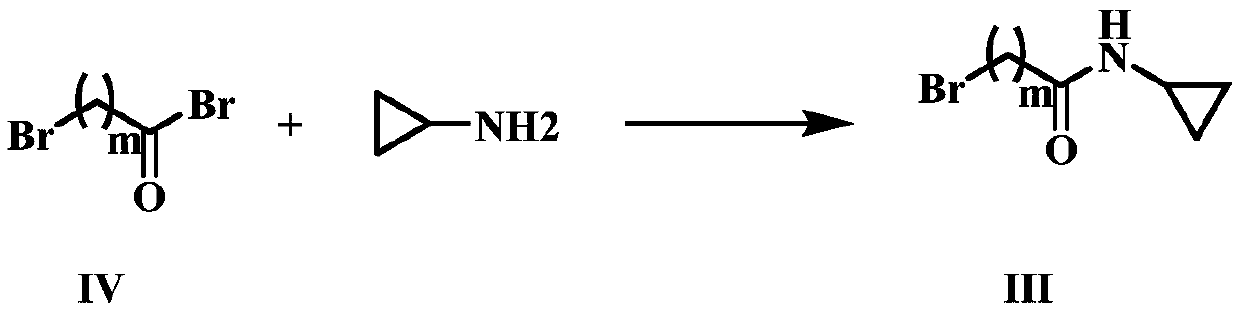Niclosamide cyclo-propyl derivative as well as preparation method and application thereof
A technology of drugs and compounds, applied in the field of medicine and chemical industry, to achieve the effect of simple and safe reaction process, good therapeutic effect and good therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] This embodiment provides compound IIIa and its preparation method:
[0038]
[0039] The concrete preparation process of above-mentioned compound IIIa is:
[0040] Under ice-water bath conditions, cyclopropylamine (1.47mmol) was dissolved in dichloromethane (2mL), triethylamine 0.21mL (1.47mmol) was added, compound Iva bromoacetyl bromide (1.47mmol) was slowly added dropwise, and stirred for 15min. After the reaction was completed, water (6 mL) was added to the reaction solution, extracted with dichloromethane (3×3 mL), the organic phases were combined, washed with saturated brine (1×3 mL), dried over anhydrous sodium sulfate, and the solvent was distilled off to obtain a residue Compound IIIa was obtained by silica gel column chromatography. Compound IIIa 1 H NMR (300MHz, CDCl 3 )δ: 6.66(s, 1H), 3.83(d, J=2.2Hz, 2H), 2.71(tq, J=7.2, 3.8Hz, 1H), 0.91~0.67(m, 2H), 0.55(ddd, J =6.9, 5.3, 3.8Hz, 2H).
Embodiment 2
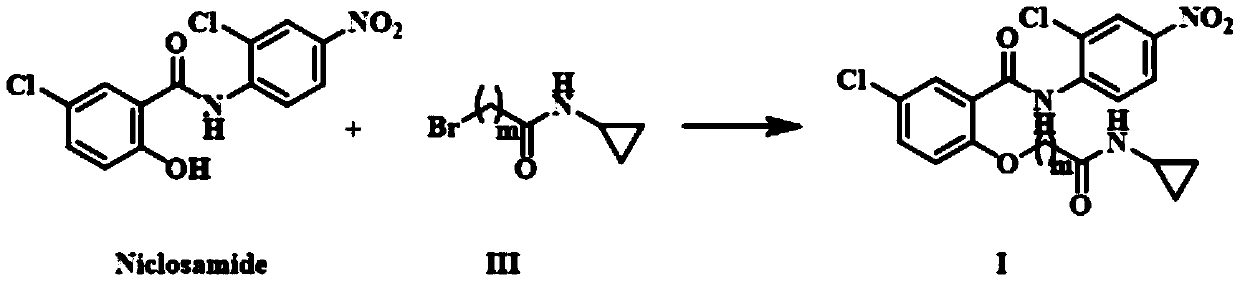
[0042] The present embodiment provides a kind of niclosamide cyclopropyl derivative Ia and preparation method thereof:
[0043]
[0044] The concrete preparation process of above-mentioned compound Ia is:
[0045]Compound IIIa (0.85mmol) prepared in Example 1 was dissolved in acetonitrile (15mL), 235mg (1.70mmol) of potassium carbonate and 277mg (0.85mmol) of niclosamide were added, and reacted for 3h. After the reaction was completed, the mixture was concentrated under reduced pressure. The residue was purified by silica gel column chromatography to obtain product Ia. Compound Ia 1 H NMR (300MHz, DMSO-d 6 )δ: 10.86(s, 1H), 8.63(d, J=9.2Hz, 1H), 8.43(d, J=2.6Hz, 1H), 8.37(s, 1H), 8.29(dd, J=9.2, 2.6 Hz,1H),7.93(d,J=2.8Hz,1H),7.66(dd,J=9.0,2.8Hz,1H),7.19(d,J=9.0Hz,1H),4.88(s,2H), 2.63(dq, J=7.1, 3.5Hz, 1H), 0.61(dd, J=7.1, 2.3Hz, 2H), 0.47~0.24(m, 2H). HR-MS (ESI) m / z: Calcdfor C 18 h 15 Cl 2 N 3 o 5 Na{[M+Na] +} 446.0286, found 446.0278.
Embodiment 3
[0047] The present embodiment provides a kind of niclosamide cyclopropyl derivative Ia and preparation method thereof:
[0048]
[0049] The concrete preparation process of above-mentioned compound Ia is:
[0050] Compound IIIa (0.85mmol) prepared in Example 1 was dissolved in dichloromethane (15mL), 172mg (1.70mmol) of triethylamine and 277mg (0.85mmol) of niclosamide were added, and reacted for 3h. Concentrate under reduced pressure, and the residue is purified by silica gel column chromatography to obtain product Ia.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com