A semicyanine compound based on benzothiazole-linked heterocycle and its preparation method and application
A compound, alkylation reaction technology, applied in chemical instruments and methods, organic chemistry, fluorescence/phosphorescence, etc., can solve the problems of inability to meet the research needs of scientific researchers, complex synthesis process, insufficient number of probes, etc. The effect of generalization, low cytotoxicity, and high quantum yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
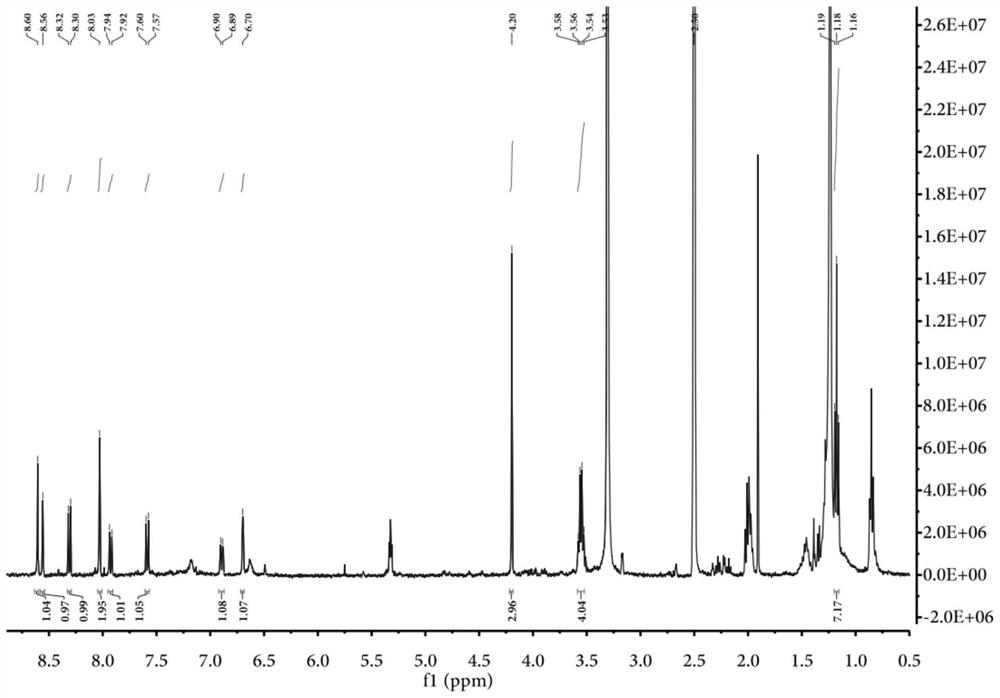
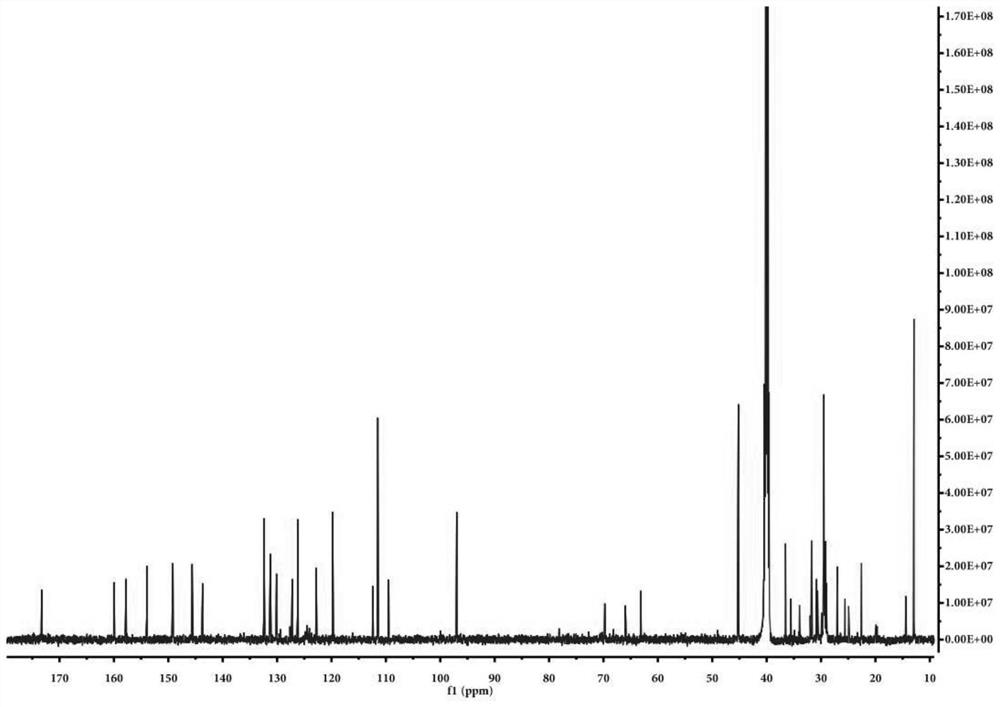
[0078]Example 1, Preparation of the compound of formula A and fluorescent probe specific identification nucleic acid double-stranded structure
[0079]First, the synthesis of the probe of formula A
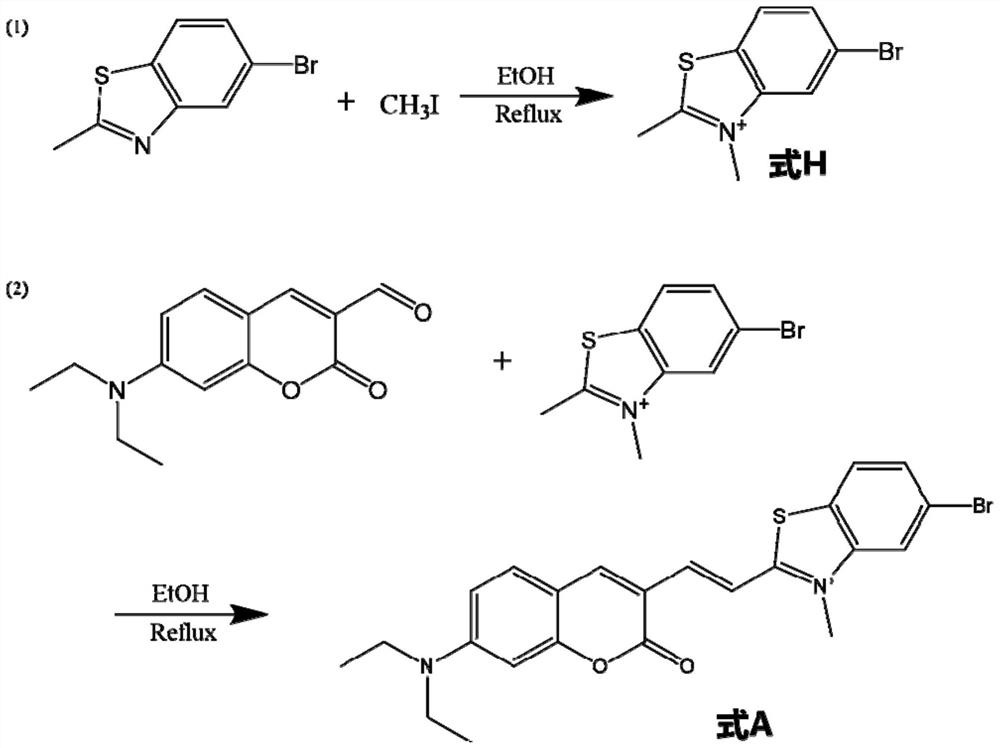
[0080]Such asfigure 1 As shown, synthesized as follows:
[0081]1, 227 mg (1.0 mmol) of 5-bromo-2-methylpain thiazole was added to 25 ml of a single neck round bottom flask equipped with 10 ml of ethanol. After preheating at 78-85 ° C, 124.5 μL (2.0 mmol) of iodide methane was added, and the heat was refluxed for 12 h, and the solution was stopped from light yellow transparent to orange yellow. Cold until the room temperature was distilled and distilled, that is, the formula H molecule was obtained.
[0082]
[0083]2. 36.3 mg (0.15 mmol) HE molecules were added to 10 ml of a 10 ml of a single neck round bottom flask equipped with 2 ml of ethanol. After preheating at 78-85 ° C for 5 min, 24.5 mg (0.1 mmol) coumarin derivative (shown in the following IV), continued to heat and reflux 12h, and the solut...
Embodiment 2
[0108]Example 2, fluorescent probe specifically recognized nucleic acid double-stranded structure
[0109]First, the synthesis of the probe described in Formula B
[0110]36.3 mg (0.15 mmol) of H molecules were added to 10 ml of a single neck round bottom flask equipped with 2 ml of ethanol. After preheating at 78-85 ° C for 5 min, 27.3 mg (0.1 mmol) 5-bromo-2,2'-joint thiophene -5'-formaldehyde was continued to heat and reflux 12h, and the solution turned from light yellow to dark red, cold Decapmetal was distilled off after room temperature to give the crude product.
[0111]
[0112]The crude product B is dissolved in dichloromethane and passed through silica gel chromatography (CH2CL2 / MeOH, V / V, 30: 1 was purified, resulting in a compound of orange red solid B molecule (32 mg, 64.4% yield). The compound is then purified by high performance liquid chromatography. The high-performance liquid chromatography separation purification step is the chromatographic column as the PROMISIL-C18 colum...
Embodiment 3
[0121]Example 3, Fluorescent Probe specifically recognized Nucleic acid G-tetracene structure
[0122]First, the synthesis of the probe described in formula C
[0123]36.3 mg (0.15 mmol) of H molecules were added to 10 ml of a single neck round bottom flask equipped with 2 ml of ethanol. After preheating at 78-85 ° C, 22.3 mg (0.1 mmol) N-ethylcarbazole-3-formaldehyde was added, continued to heat generation 12h, and the solution was stopped from light yellow to orange red, cold to room temperature, decompression distillation To obtain a crude product.
[0124]The crude product C is dissolved in dichloromethane and passed silica gel chromatography (CH2CL2 / MeOH, V / V, 30: 1) Purified to obtain a compound of orange red solid C molecule (39 mg, 87.1% yield). The compound is then purified by high performance liquid chromatography. The high-performance liquid chromatography separation purification step is the chromatographic column as the PROMISIL-C18 column (250 mm × 4.6 mm, 20 μm), and the flo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com