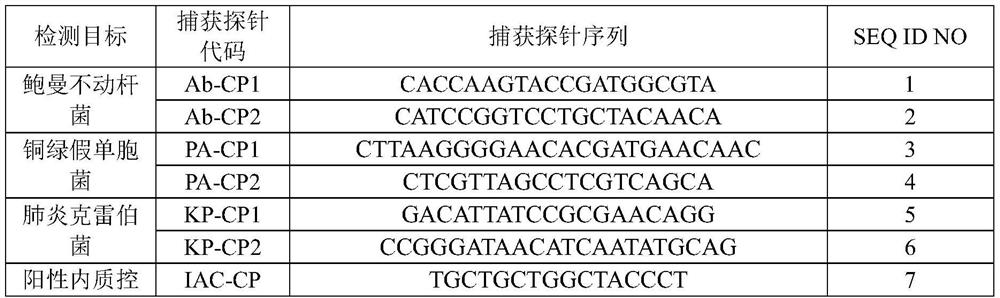
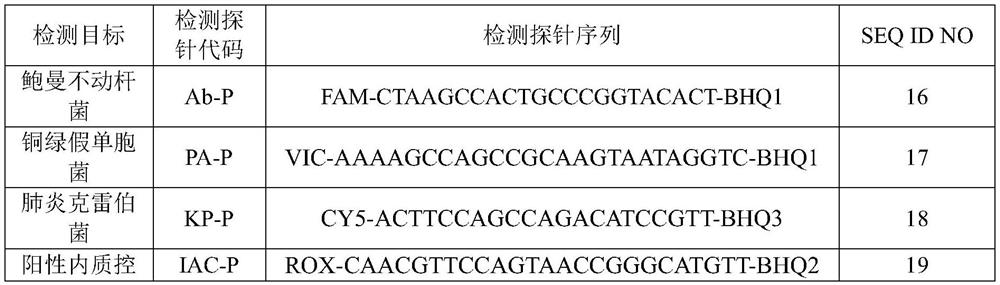
Nucleic acid reagent, kit, system and method for detecting pathogenic bacteria of bloodstream infection
A technology of pathogenic bacteria and kits, applied in the biological field, can solve the problems of easy contamination of PCR, long detection cycle, and low nucleic acid content of pathogenic bacteria, achieve high conservatism and specificity, reduce the risk of contamination, and increase the effect of nucleic acid concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Embodiment 1 detection method and detection result judgment
[0064] 1. Preparation of streptavidin magnetic beads
[0065] (1) Magnetic bead pretreatment: Take 10-30 μL of mixed amino magnetic beads (10 mg / mL) into EP tube, magnetically separate, then wash with PBS buffer 1-2 times, magnetically separate to remove the supernatant, Obtain the first magnetic bead precipitation;
[0066] (2) Glutaraldehyde activation: Add 100-200 μL of freshly prepared glutaraldehyde solution (15-25%) to the first magnetic bead pellet, mix well, and react for 1-2 hours in the dark at 25°C for activation;
[0067] (3) Washing after activation: after activation of the magnetic beads, magnetically separate, wash 1-2 times with PBS buffer, remove the supernatant by magnetic separation, and obtain the second magnetic bead precipitate;
[0068] (4) Coupling: Add 5-20 μg streptavidin to the second magnetic bead precipitation, mix well, and react at 25°C for 2-3 hours in the dark for coupling; ...
Embodiment 2
[0099] Embodiment 2 minimum detection limit verification
[0100] Use a concentration of 10 5 Copy / μL nucleic acids of Acinetobacter baumannii, Pseudomonas aeruginosa, and Klebsiella pneumoniae were mixed in equal proportions as templates, and were serially diluted to 10 3 copies / μL, 10 2 copies / μL, 10 copies / μL, 2 copies / μL and 1 copy / μL, as templates for evaluation.
[0101] According to the detection method of Example 1, the evaluation templates of each concentration were detected respectively, and the detection was repeated 20 times for each concentration gradient, and the average value was taken as the final detection result, as shown in Table 4.
[0102] Table 4
[0103]
[0104] It can be seen from Table 4 that the minimum detection limit of the target genome of the bloodstream infection pathogenic bacteria detected by the disclosed kit reaches 1 copy / μL, and the detection sensitivity of the disclosed kit is relatively high.
Embodiment 3
[0105] Example 3 specificity verification
[0106]Select bacteria that are similar to the target pathogen species and live in the same environment, including Escherichia coli (purchased from the National Culture Collection Center, number CVCC2356), Streptococcus pneumoniae (purchased from the National Culture Collection Center, number CVCC4105) ), Staphylococcus aureus (purchased from the National Culture Collection Center, numbered CVCC1882), Enterococcus faecalis (purchased from the National Culture Collection Center, numbered CVV3936), Salmonella (purchased from the National Culture Collection Center, numbered CVCC3949 ), Enterobacter cloacae (purchased from China Medical Bacteria Collection Management Center, No. CMCC45301), Candida albicans (purchased from National Culture Collection Center, No. CVU0037) etc. as specificity evaluation samples, using the kit provided by this disclosure According to the detection method of Example 1, the above-mentioned specificity evaluati...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com