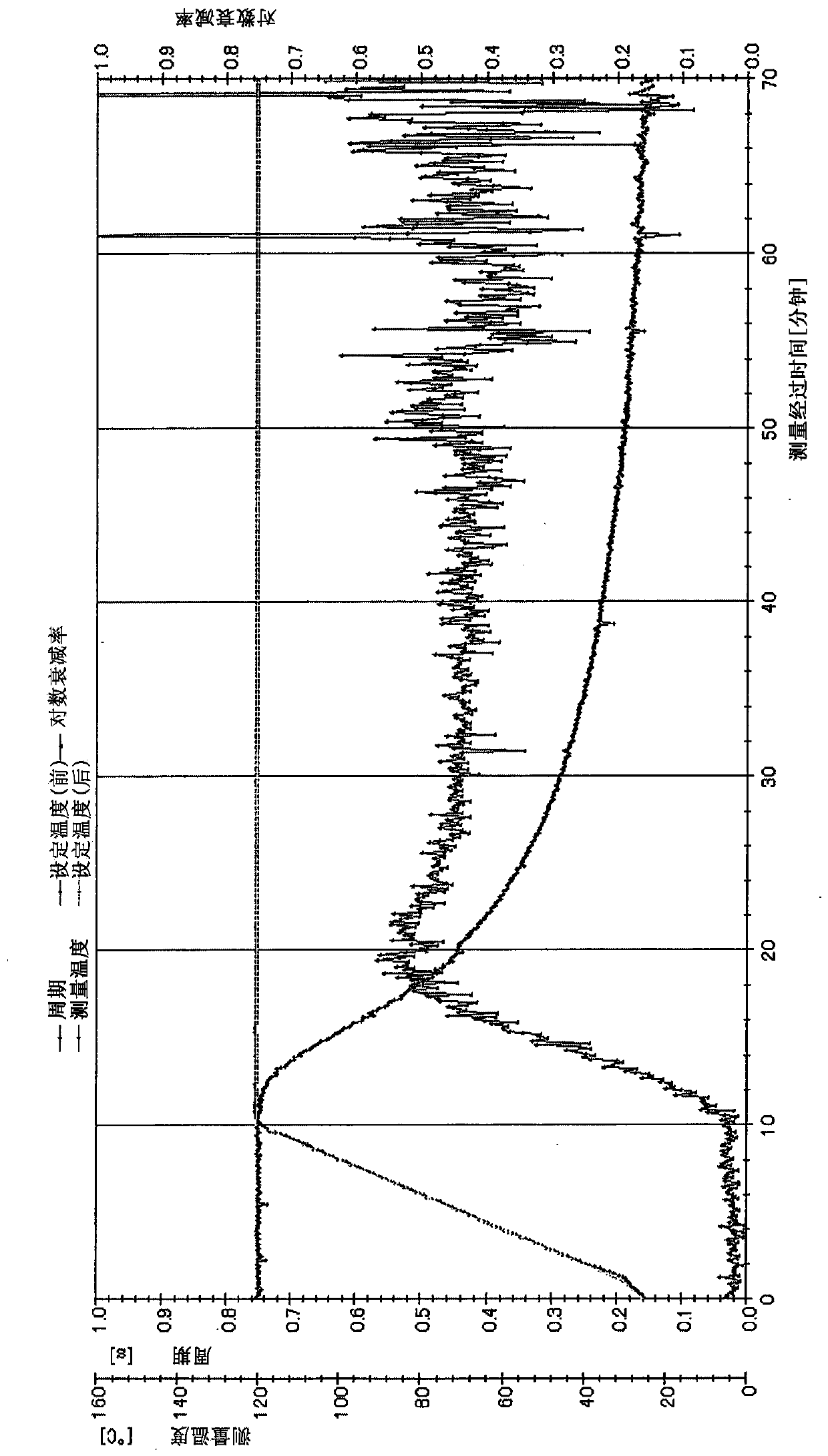
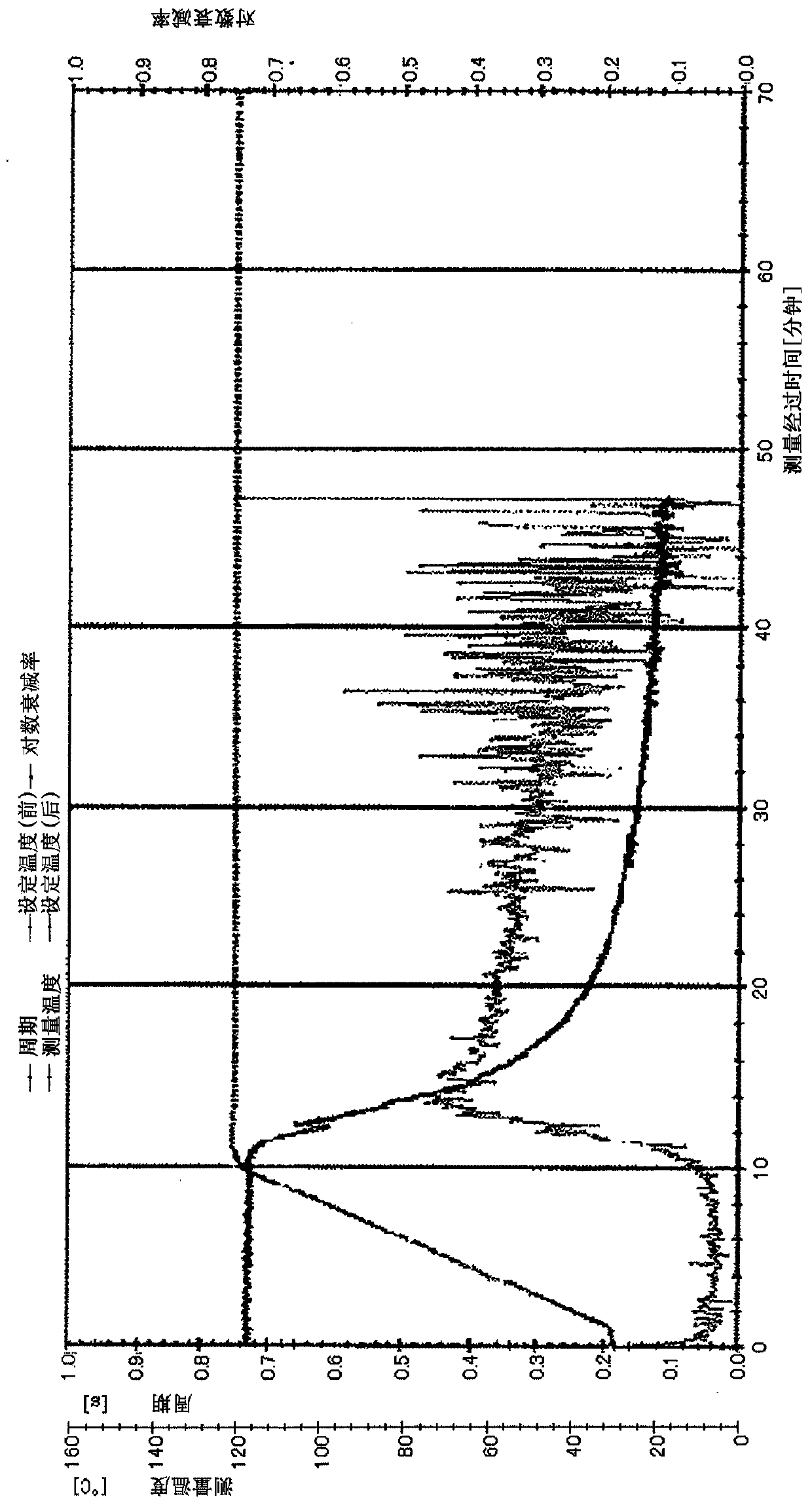
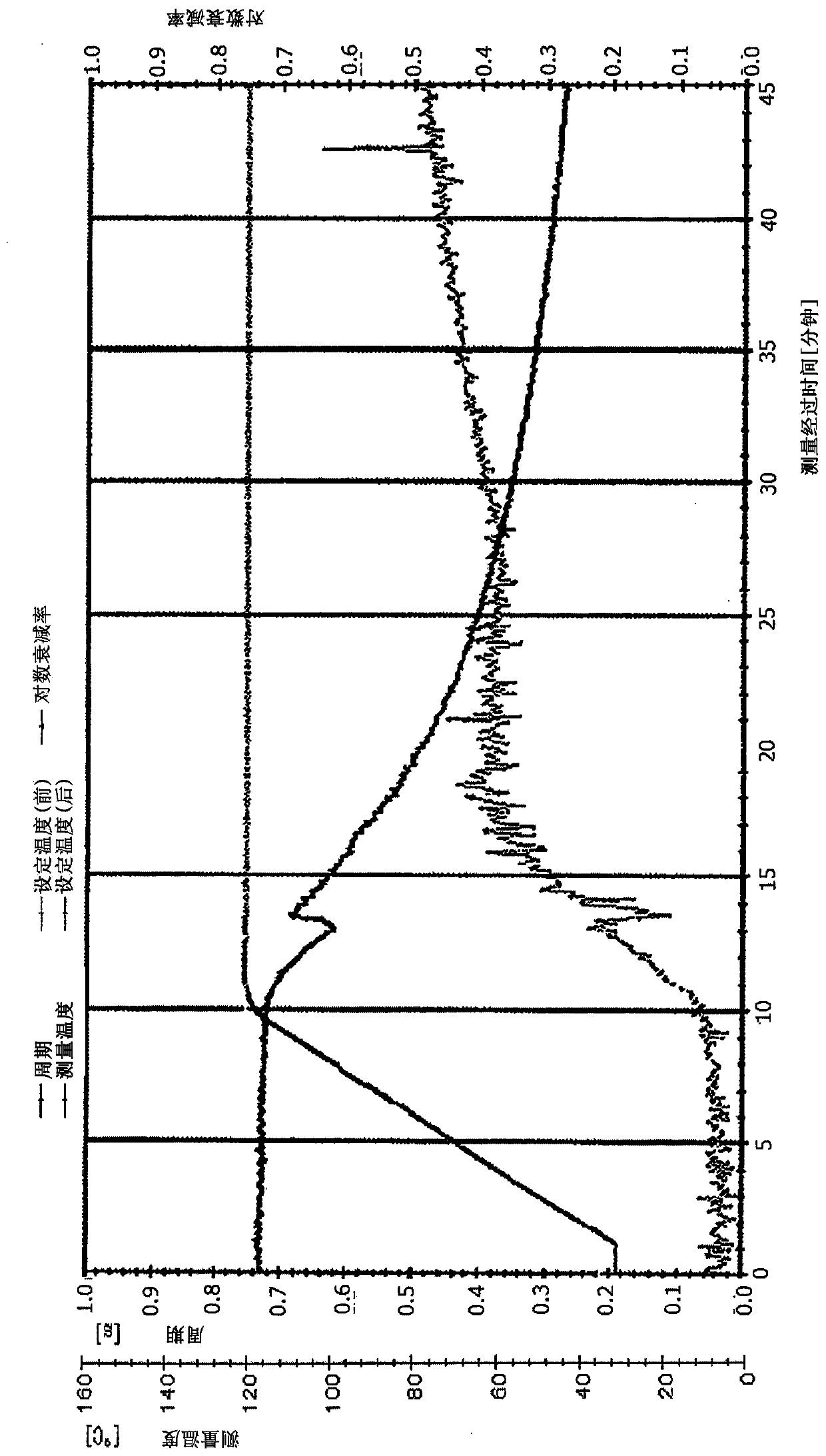
Unsaturated group-containing ester compound, polymer, thermosetting resin composition, and cured film
A technology of resin composition and ester compound, applied in the direction of organic chemistry, coating, etc., can solve the problem of slow curing reaction speed, and achieve the effect of speeding up the reaction speed, good curing performance, and excellent low-temperature curability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0291] Hereinafter, the present invention will be described in further detail based on examples. It should be noted that the present invention is not limited to the following examples. It should be noted that parts in the text indicate weight.
Synthetic example 1
[0293] Mix 54 parts of ethylene glycol monoacetoacetate monomethacrylate, 58 parts of tert-butyl acrylate, 38 parts of potassium carbonate, 2 parts of 18-crown-6-ether, and 112 parts of tetrahydrofuran, and stir at 50°C for 3 hour. After the reaction was completed, cyclohexane and water were added and washed with water. After the organic layer was neutralized with a saturated aqueous ammonia chloride solution, it was washed twice with water, and the obtained organic layer was concentrated under reduced pressure to obtain monomer A.
Synthetic example 2
[0295] Mix 54 parts of ethylene glycol monoacetoacetate monomethacrylate, 32 parts of tert-butyl acrylate, 38 parts of potassium carbonate, 2 parts of 18-crown-6-ether, and 112 parts of tetrahydrofuran, and stir at 50°C for 3 hour. After the reaction was completed, cyclohexane and water were added and washed with water. After the organic layer was neutralized with a saturated aqueous ammonia chloride solution, it was washed twice with water, and the obtained organic layer was concentrated under reduced pressure to obtain monomer B.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| hydroxyl value | aaaaa | aaaaa |
| weight-average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com