The method for preparing orlistat intermediate
An orlistat and intermediate technology, which is applied in the field of biosynthesis of -β-hydroxytetradecanoic acid ester compounds, can solve the problems of affecting ee value, existing catalysts, and difficulty in guaranteeing process stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
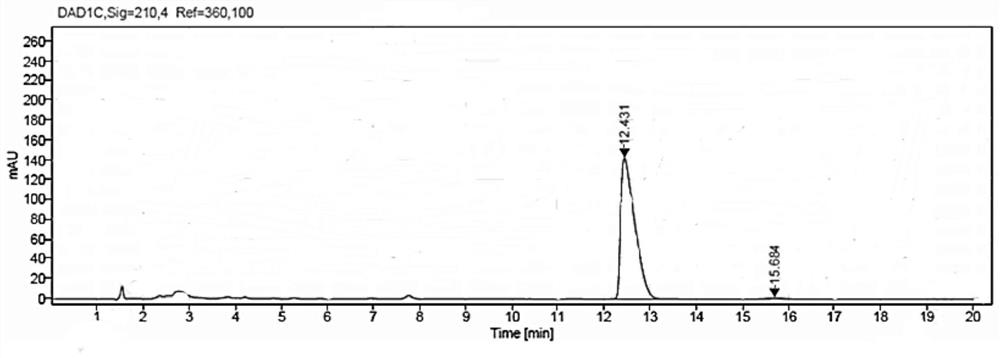
[0071] Weigh 1g of methyl β-carbonyltetradecanoate and 1.5g of glucose into a 100mL three-neck flask, then add 50mL of PBS buffer solution with a concentration of 50mM and a pH value of 7.0; put the three-necked flask into a constant temperature water bath , the stirring speed was adjusted to 800rpm, the temperature was 35°C, and then 10mg NADP was added + , 25mg of glucose dehydrogenase GDH enzyme powder (shown in SEQ ID NO:3), and 100mg of ketoreductase JR3789 enzyme powder (shown in SEQ ID NO:1), to obtain a mixed reaction solution, adjust the pH with 2M NaOH solution The temperature was maintained at 7.0-7.5, and the temperature was maintained at 35° C., and the reaction progress was monitored by HPLC. After 9 hours, the reaction was completed, and the conversion rate was measured to be >99%.
[0072] After the reaction, first raise the temperature to 60°C and keep it warm for 15min, then cool down to 20-25°C, add 80mL of ethyl acetate to extract and stir for 20min, filter...
Embodiment 2
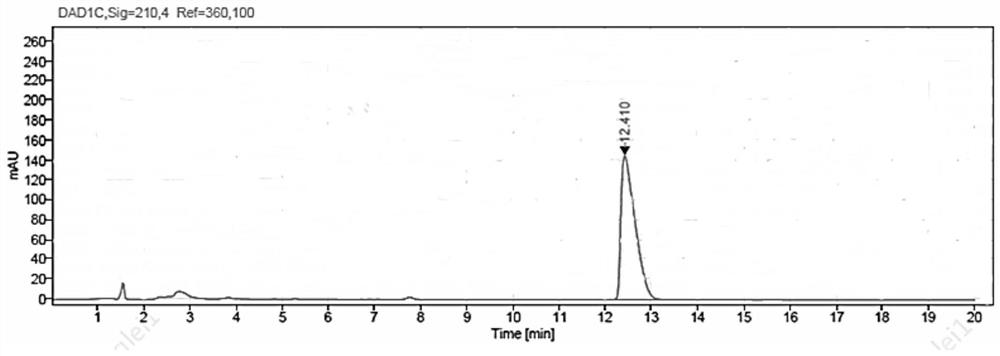
[0074] Weigh 1g of ethyl β-carbonyltetradecanoate and 1.5g of glucose into a 100mL three-necked flask, and then add 50mL of PBS buffer solution with a concentration of 50mM and a pH value of 7.0; put the three-necked flask into a constant temperature water bath , the stirring speed was adjusted to 900rpm, the temperature was 35°C, and then 10mg NADP+, 35mg glucose dehydrogenase GDH enzyme powder (shown in SEQ ID NO:3), and 150mg ketoreductase JR3789 enzyme powder (shown in SEQ ID NO:1) were added respectively Shown), to obtain a mixed reaction solution, adjust the pH with 2M NaOH solution to maintain between 7.0-7.5, the temperature is maintained at 35 ° C, using HPLC to monitor the reaction process, 10h after the end of the reaction, and measured conversion> 99%.
[0075] After the reaction, first raise the temperature to 60°C and keep it warm for 15min, then cool down to 20-25°C, add 80mL of ethyl acetate to extract and stir for 20min, filter, and separate the filtrate to tak...
Embodiment 3
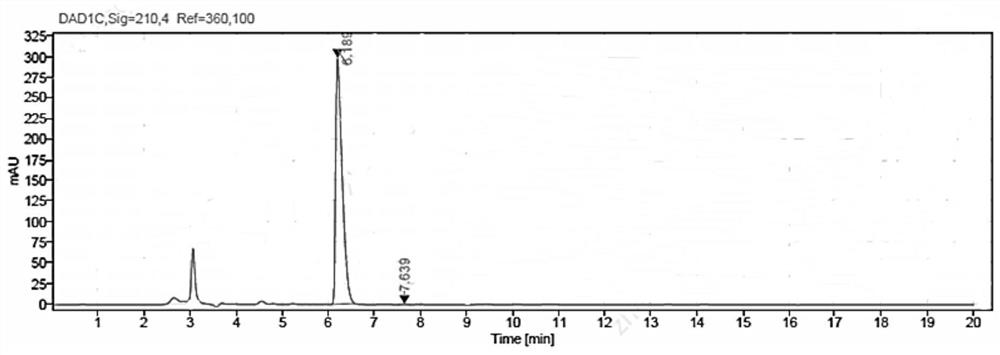
[0077] Weigh 5g of methyl β-carbonyltetradecanoate and 7.5g of glucose into a 100mL three-necked flask, then add 50mL of PBS buffer solution with a concentration of 50mM and a pH value of 7.0; put the three-necked flask into a constant temperature water bath , the stirring speed was adjusted to 800rpm, the temperature was 35°C, and then 50mg NADP+, 125mg glucose dehydrogenase GDH enzyme powder (shown in SEQ ID NO:3), and 500mg ketoreductase JR3789 enzyme powder (shown in SEQ ID NO:1) were added respectively Shown), the mixed reaction solution was obtained, the pH was adjusted with 2M NaOH solution to maintain between 7.0-7.5, the temperature was maintained at 35 ° C, the reaction progress was monitored by HPLC, the reaction was completed after 13 hours, and the conversion rate > 99% was measured.
[0078] After the reaction, first raise the temperature to 60°C and keep it warm for 15min, then cool down to 20-25°C, add 80mL of ethyl acetate to extract and stir for 20min, filter,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com