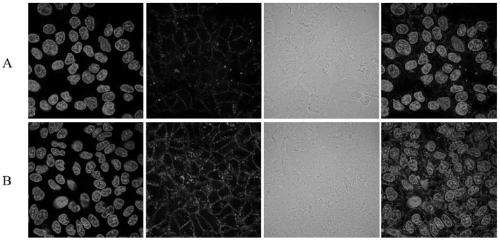
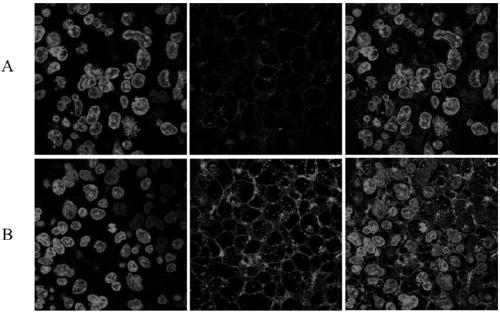
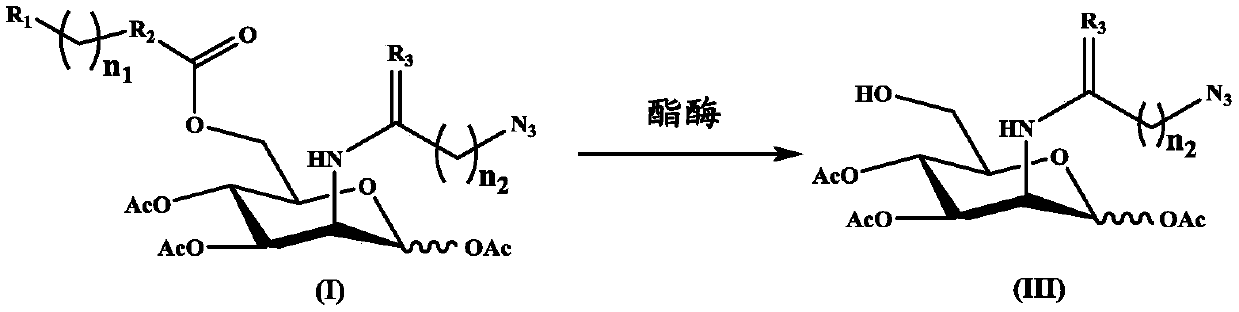
N-azido acetyl-D-mannosamine derivative, preparation method thereof, and application of N-azido acetyl-D-mannosamine derivative in esterase detection
A technology of azidoacetyl and mannosamine, which is applied in the preparation of sugar derivatives, sugar derivatives, sugar derivatives, etc., to achieve high specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0059] Preparation of compound (III-1)
[0060]
[0061] Under ice-water bath, compound (IV-1) (0.75g, 1.5mM) was dissolved in anhydrous tetrahydrofuran, and then a tetrahydrofuran solution of 1M tetrabutylammonium fluoride (1.5eq., 2.25mM) was added, and then in The reaction was stirred at room temperature for 3 hours. After the reaction was completed, the reaction was quenched with 10 mL of a saturated aqueous solution of ammonium chloride, extracted with ethyl acetate (50 mL×3), the organic layer was collected, dried over anhydrous sodium sulfate, filtered, and the solvent was removed. The crude product was purified by silica gel column chromatography, and the eluent was petroleum ether / ethyl acetate (v / v=1:2~1:3) to obtain a white solid (541 mg, 93%).
Embodiment 1
[0063] Preparation of compound (1)
[0064]
[0065] Under the protection of an ice-water bath and argon, the compound of formula (III-1) (388 mg, 1 mM) was dissolved in 15 mL of a dry mixed solution of tetrahydrofuran / dichloromethane (THF / DCM, 1:1 v / v), and then added Triethylamine (0.809g, 8mM) and N,N-diisopropylethylamine (1.034g, 8mM) were reacted for 10 minutes, and dissolved in 10mL of dry THF / DCM (1:1v / v) was added dropwise 2-(furan-2-yl)ethyl chloroformate (696 mg, 4 mM) in solution. Continue to react at 0°C for 1 hour, remove the ice-water bath, return to room temperature, and react for 3 hours. After the reaction was complete, the solvent was removed, and the crude product was purified by silica gel column chromatography with petroleum ether / ethyl acetate (v / v=1:1) as the eluent to obtain a white solid (189 mg, 36%). 1 H NMR (400MHz, CD 3 OD): δ7.511(d,1H),6.362(m,1H),6.125(d,1H),5.983(s,1H),5.328-5.098(m,4H),4.733(s,1H),4.359 -4.280(m,1H),4.150-4.070(m,2H),3...
Embodiment 2
[0067] Preparation of compound (2)
[0068]
[0069] Under the protection of an ice-water bath and argon, the compound of formula (III-1) (388 mg, 1 mM) was dissolved in 15 mL of a dry mixed solution of tetrahydrofuran / dichloromethane (THF / DCM, 1:1 v / v), and then added Triethylamine (0.809g, 8mM) and N,N-diisopropylethylamine (1.034g, 8mM) were reacted for 10 minutes, and dissolved in 10mL of dry THF / DCM (1:1v / v) was added dropwise solution of benzyl chloroformate (680 mg, 4 mM). Continue to react at 0°C for 1 hour, remove the ice-water bath, return to room temperature, and react for 3 hours. After the reaction was complete, the solvent was removed, and the crude product was purified by silica gel column chromatography with petroleum ether / ethyl acetate (v / v=1:1) as the eluent to obtain a white solid (198 mg, 38%). 1 H NMR (400MHz, CD 3 OD):δ7.32-7.35(d,5H),5.997(s,1H),5.330-5.090(m,4H),4.725(s,1H),4.356-4.281(m,1H),4.150-4.073( m,2H), 3.936(s,2H), 2.173-1.910(m,9H).ESI...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com