Polyiodinated aromatic acid modified Andersen polyacid organic derivatives and application thereof as CVB3 virus inhibitor
A technology of aromatic acids and derivatives, applied in antiviral agents, 7/17 group organic compounds without C-metal bonds, organic chemistry, etc., to achieve good anti-CVB3 virus, easy synthesis, and enhanced cell survival rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: A 6 、A 7 preparation of
[0033] Andersen Polyacid Organic Derivative A Modified with Polyiodoaromatic Acids 6 The molecular formula is (TBA) 3 [MnMo 6 o 18 ((OCH 2 ) 3 CNHCOC 6 h 2 -2-OH-3,5-I 2 ) 2 ], wherein the cation is TBA, and TBA is [(N(C 4 h 9 ) 4 )] + , the structure of its anion is as follows:
[0034]
[0035] A 6 For the preparation, refer to the method of the literature Inorg.Chem. (2016, 55, 9497–9500), specifically, the reaction of trishydroxymethylaminomethane and 2-hydroxyl-3,5-diiodobenzoyl chloride to prepare the corresponding amide complex body, and then the obtained amide ligand is refluxed with octamolybdic acid and trivalent manganese acetate, and the obtained filtrate is diffused in ether to obtain A 6 .
[0036] Anderson polyacid organic derivatives modified with polyiodoaromatic acids (A 7 ) molecular formula is (TBA) 3 [MnMo 6 o 18 -((OCH 2 ) 3 CNHCOC 6 h 2 -2,3,5-I 3 ) 2 ], wherein the cation is TBA,...
Embodiment 2
[0039] Embodiment 2: Andersen polyacid organic derivative A modified by polyiodoaromatic acid 6 、A 7 Toxicity to host Hep-2 cells
[0040] Hep-2 cells were plated in 96-well plates at 37°C, 5% CO 2 After the incubator grows a monolayer, discard the cell culture medium, and add different concentrations of A 6 and A 7 After 48 hours, the cytotoxicity was recorded under a microscope, and the cell viability was measured by MTT method. The specific steps of the MTT method are as follows: add 30 μL of MTT (5 mg·mL -1 ), after incubation for 3-4 h, the supernatant was removed, and 50 μL of DMSO was added to dissolve the precipitate. Read the corresponding absorbance (OD) at 492 nm with a microplate reader 492 value).
[0041] SPSS 11.5 software was used to calculate the median toxic concentration (Median cyctoxic concentration, CC50) of the drug on the cells.
[0042] Cell viability=(average OD of drug group 492 Value / average OD of cell control group 492 value)×100%
Embodiment 3
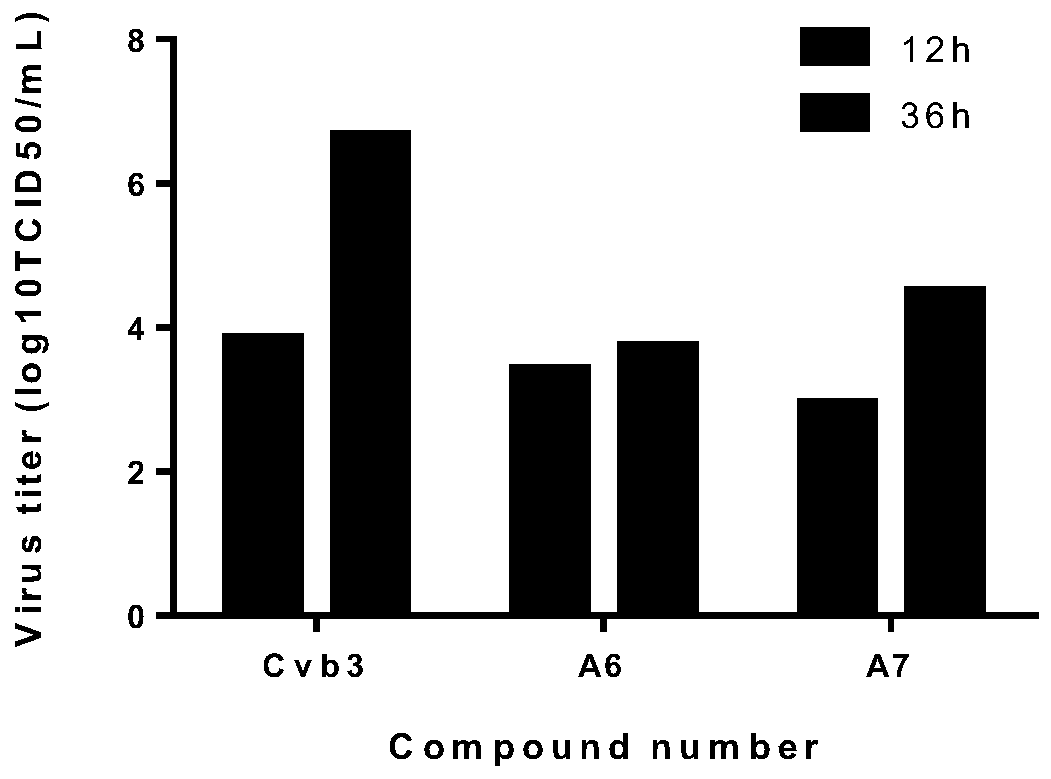
[0043] Embodiment 3: Andersen polyacid organic derivative A modified by polyiodoaromatic acid 6 、A 7 Inhibitory activity against CVB3
[0044] Hep-2 cells were plated in 96-well plates at 37°C, 5% CO 2 After the monolayer was grown in the incubator, discard the culture medium, infect the cells with 100TCID50 CVB3 virus solution for 1h, and add different concentrations (2.5μg / mL, 5μg / mL, 10μg / mL, 20μg / mL, 40μg / mL, 80μg / mL) of compound A 6 、A 7 (ribavirin as a positive control drug) to incubate the cells. After continuing to culture for about 48 hours, when about 90% of the CPE lesions appeared in the virus control wells, the cytopathic effect (CPE) was observed under a microscope. Observation and recording method of CPE: No cytopathic disease is marked as -, cytopathic disease of less than 25% is recorded as +, 25%-50% cytopathic disease is recorded as ++, 50%-75% cytopathic disease is recorded as +++, more than 75% Cytopathy was recorded as ++++.
[0045] After the obs...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com