Efficient preparation method of 1-aryl-4-butene compound
A compound and aryl technology, applied in the field of fine chemicals and related chemistry, to achieve the effects of high yield, easy operation and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1: the synthesis of p-methyl-3-butenylbenzene
[0048] In a 25mL reactor, add 4-methylbenzyl chloride (0.028g, 0.2mmol), allylboronic acid pinacol ester (0.067g, 0.4mmol), palladium acetate (2.2mg, 0.01mmol), cesium fluoride (0.091g, 0.6mmol), 4,5-bisdiphenylphosphine-9,9-dimethylxanthene (8.7mg, 0.015mmol), stirred at 80°C for 12h under nitrogen. Column chromatography separation (silica gel, 200-300 mesh; developer, petroleum ether) gave 0.023 g of p-methyl-3-butenylbenzene with a yield of 83%.
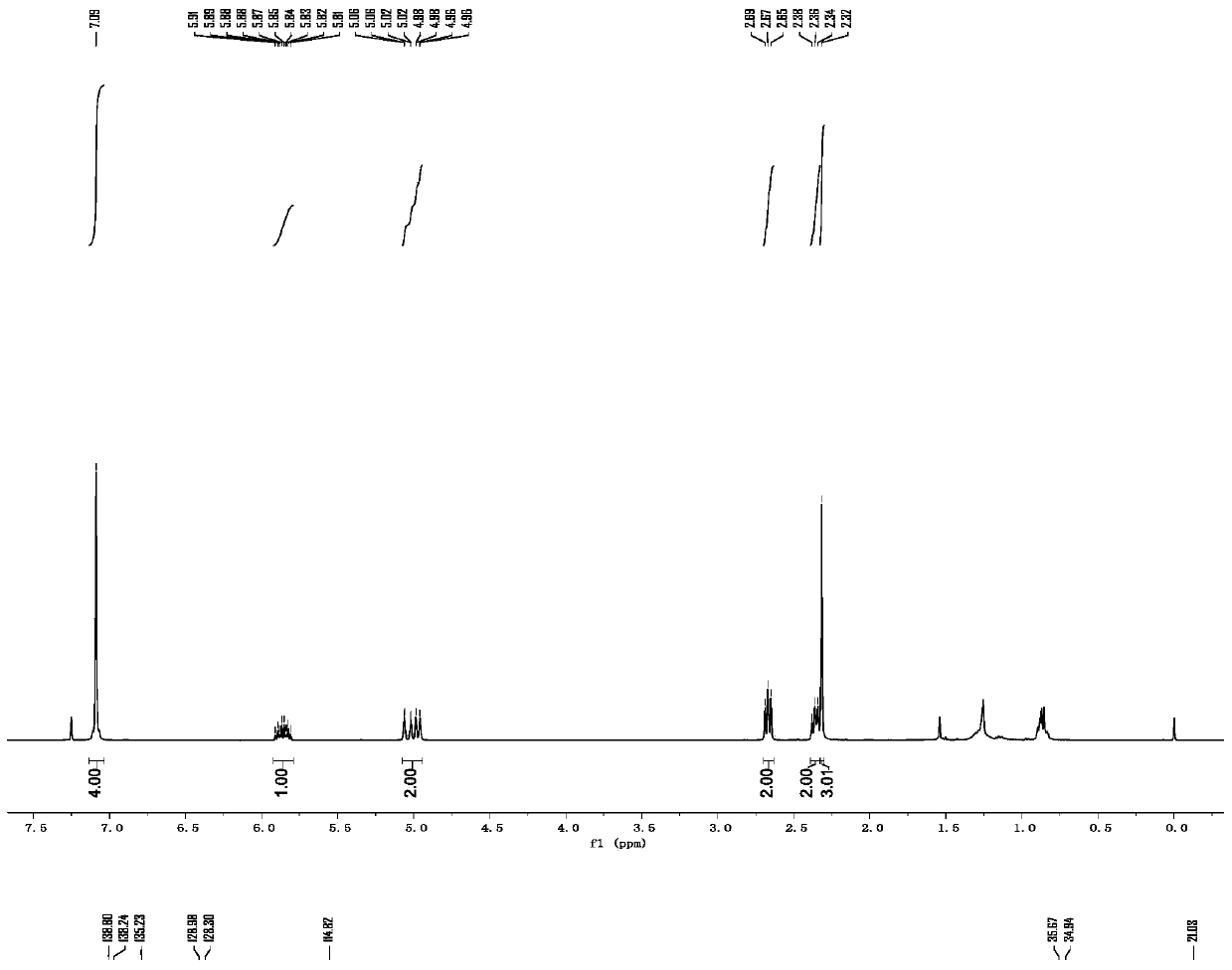
[0049] p-Methyl-3-butenylbenzene
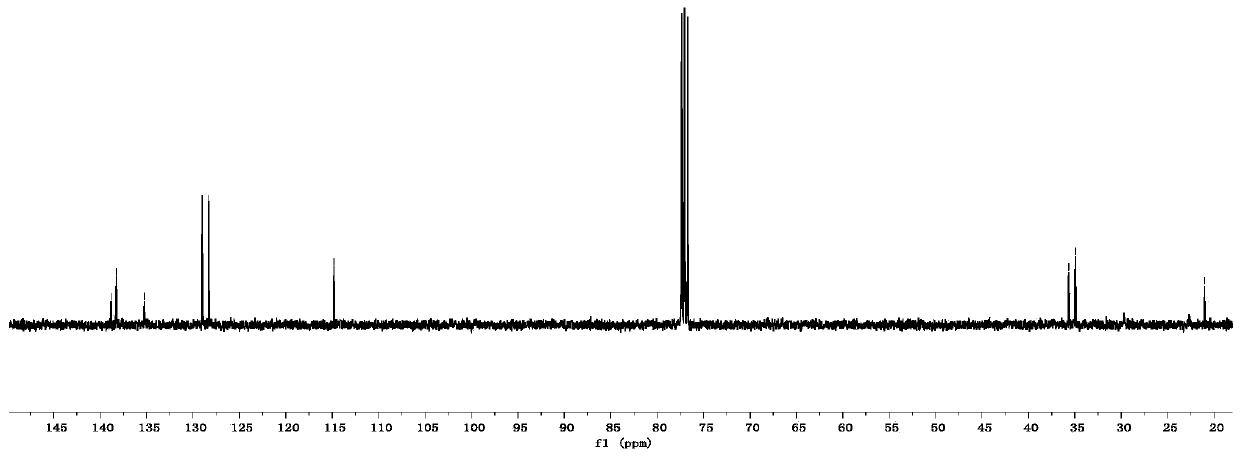
[0050] colorless oily liquid; 1 H NMR (400MHz, CDCl 3):δ7.09(s,4H),5.92–5.80(m,1H),5.08–4.94(m,2H),2.67(t,J=8.0Hz,2H),2.36(q,J=8.0Hz, 2H), 2.32(s, 3H); 13 C NMR (100MHz, CDCl 3 ): δ139.8, 138.2, 135.2, 128.9, 128.3, 114.8, 35.6, 34.9, 21.0.
Embodiment 2
[0051] Embodiment 2: the synthesis of p-ethyl-3-butenylbenzene
[0052] The operation was the same as in Example 1, and 0.024 g of p-ethyl-3-butenylbenzene was obtained from the reaction of 4-ethylbenzyl chloride and allylboronic acid pinacol ester, with a yield of 79%.
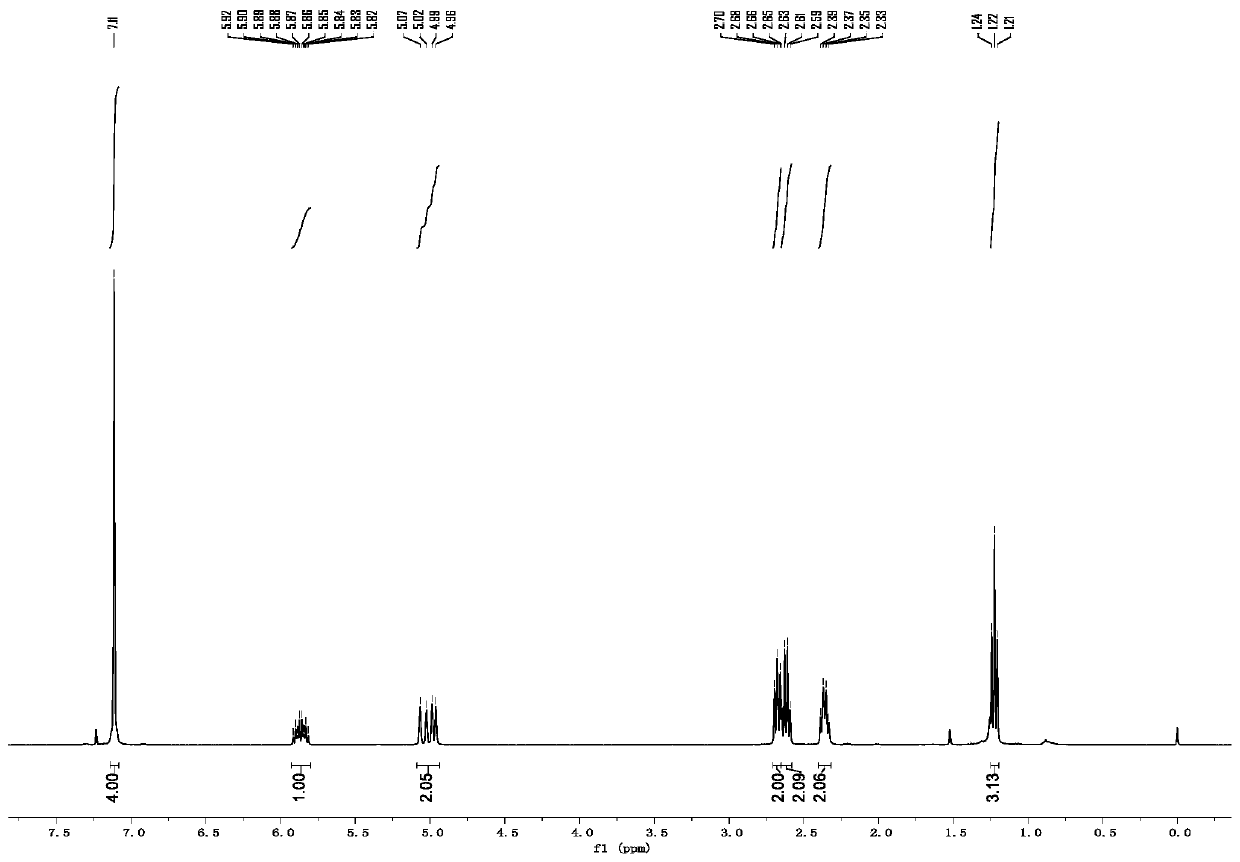
[0053] p-Ethyl-3-butenylbenzene
[0054] colorless oily liquid; 1 H NMR (400MHz, CDCl 3 ):δ7.11(s,4H),5.92–5.90(m,1H),5.09–4.95(m,2H),2.68(t,J=8.0Hz,2H),2.62(q,J=8.0Hz, 2H), 2.36(q, J=8.0Hz, 2H), 1.22(t, J=8.0Hz, 3H); 13 C NMR (100MHz, CDCl 3 ): δ141.7, 139.1, 138.3, 128.4, 127.8, 114.8, 35.6, 34.9, 28.5, 15.7.
Embodiment 3
[0055] Embodiment 3: the synthesis of p-tert-butyl-3-butenylbenzene
[0056] The operation is the same as in Example 1, and 0.030 g of p-tert-butyl-3-butenylbenzene is obtained by reacting 4-tert-butylbenzyl chloride with allylboronic acid pinacol ester, and the yield is 82%.
[0057] p-tert-Butyl-3-butenylbenzene
[0058] colorless oily liquid; 1 H NMR (400MHz, CDCl 3 ):δ7.35(d, J=8.0Hz, 2H), 7.18(d, J=8.0Hz, 2H), 5.98–5.96(m, 1H), 5.14–4.99(m, 2H), 2.76–2.69( t,J=8.0Hz,2H), 2.44–2.39(m,2H),1.36(s,9H); 13 C NMR (100MHz, CDCl 3 ): δ148.6, 138.8, 138.3, 128.1, 125.2, 114.8, 35.5, 34.8, 34.4, 31.4.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com