Metformin hydrochloride controlled-release tablet
A technology of metformin hydrochloride controlled-release tablets and metformin hydrochloride, which is applied in the direction of medical preparations, coatings, and pill delivery of non-active ingredients, which can solve the problems of taking 2 or 3 times a day and short half-life, so as to increase compliance , Reduce production costs, reduce the effect of treatment costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
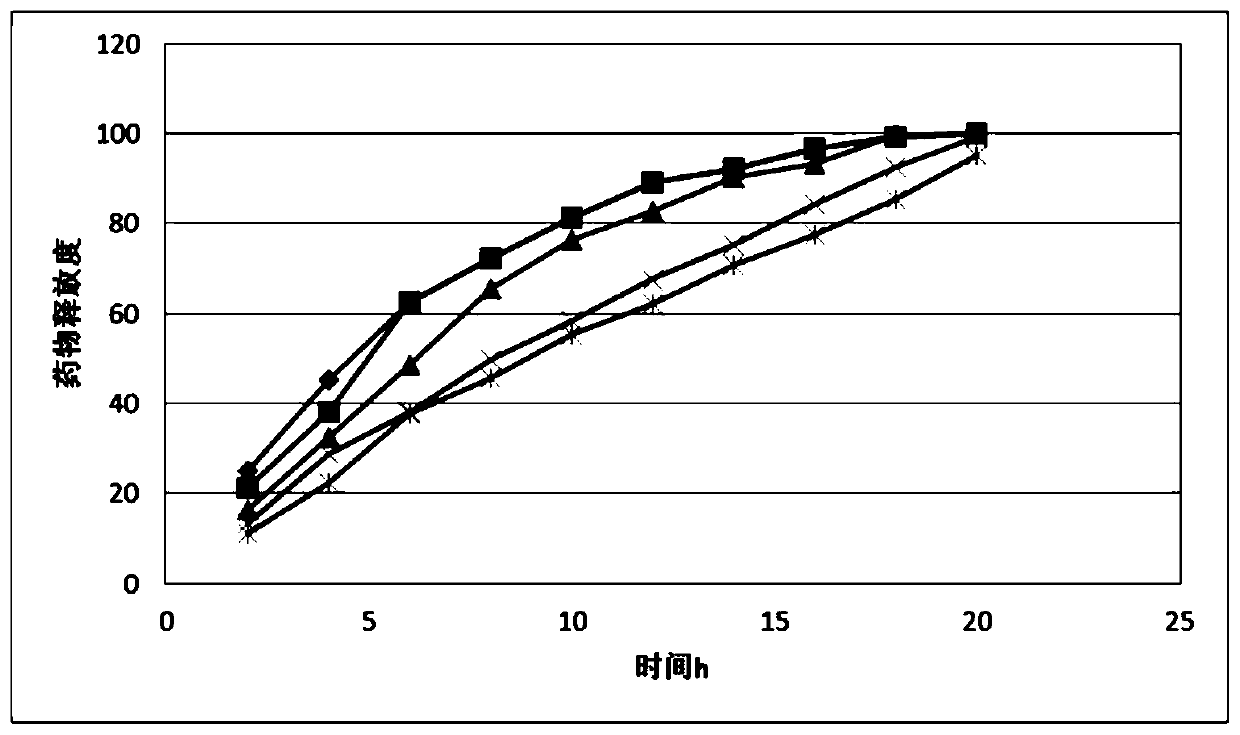
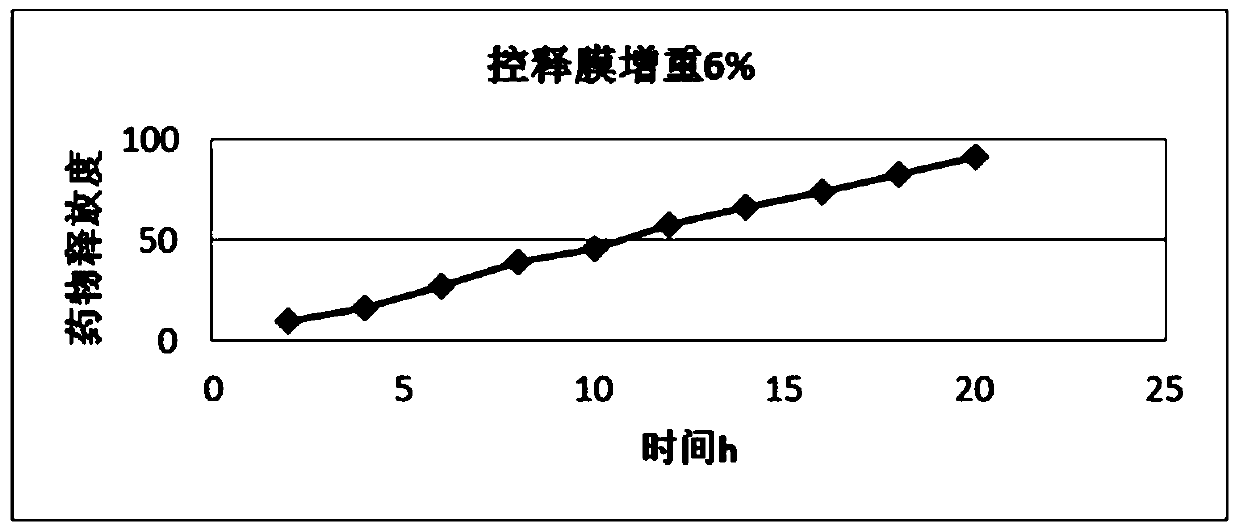
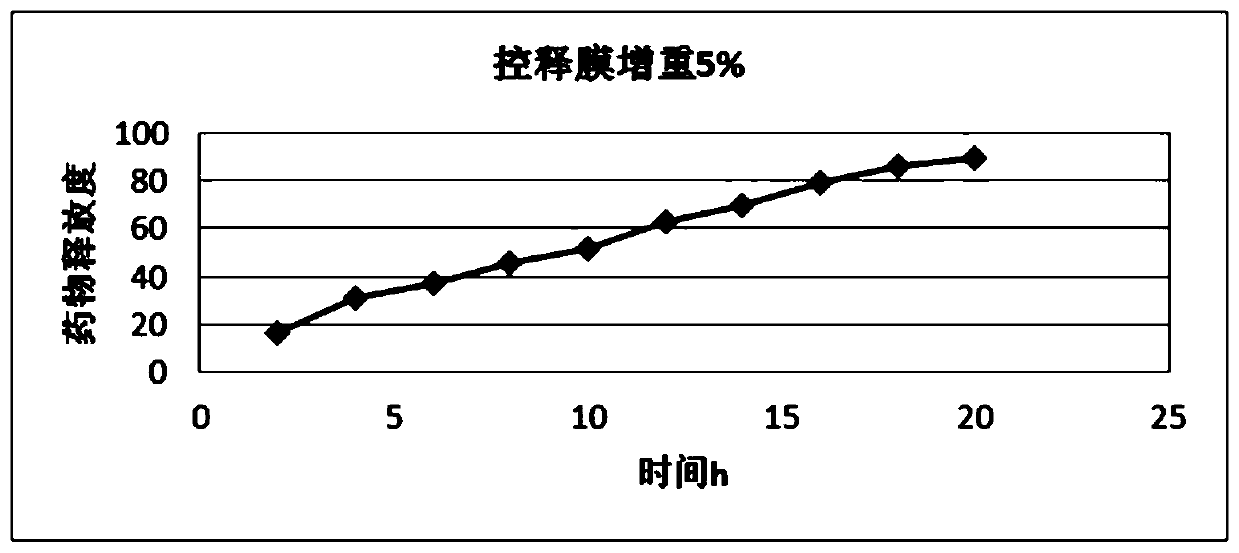
Image
Examples
Embodiment 1
[0032]
[0033] (1) Take metformin hydrochloride and pass through an 80-mesh sieve, and add the sieved metformin hydrochloride into the wet granulator;
[0034] (2) Add the fillers and binders into the wet granulator, the speed of the wet granulator is 20rpm, and spray 20% of the solid feed into purified water to granulate for 15 minutes;
[0035] (3) Dry the prepared granules in an oven at 40°C for 20 minutes, and control the moisture content of the granules at 3%;
[0036] (4) Add the dried particles into the mixer, set the speed at 60rpm, and mix for 10min;
[0037] (5) Add the lubricant into the mixer, continue mixing for 2-5 minutes, and take out the mixed materials for later use;
[0038] (6) Prepare the blank core material and purified water into a suspension, and the blank core material accounts for 25%;
[0039] (7) Spray the prepared suspension into the centrifugal granulator, set the rotation speed to 200rpm, set the spray speed to 30g / min, and set the drying t...
Embodiment 2
[0046]
[0047] (1) Take metformin hydrochloride and pass through an 80-mesh sieve, and add the sieved metformin hydrochloride into the wet granulator;
[0048] (2) Add the fillers and binders into the wet granulator, the speed of the wet granulator is 20rpm, spray 20% of the solid feed into purified water and granulate for 15min;
[0049] (3) Dry the prepared granules in an oven at 40°C for 20 minutes, and control the moisture content of the granules at 2%;
[0050] (4) Add the dried particles into the mixer, set the speed at 60rpm, and mix for 10min;
[0051] (5) Add the lubricant into the mixer, continue mixing for 2-5 minutes, and take out the mixed materials for later use;
[0052] (6) Prepare the blank core material and purified water into a suspension, and the blank core material accounts for 35%;
[0053] (7) Spray the prepared suspension into the centrifugal granulator, set the rotation speed to 200rpm, set the spray speed to 30g / min, and set the drying temperatu...
Embodiment 3
[0060]
[0061] (1) Take metformin hydrochloride and pass through an 80-mesh sieve, and add the sieved metformin hydrochloride into the wet granulator;
[0062] (2) Add the fillers and binders into the wet granulator, the speed of the wet granulator is 40pm, and spray 20% solids into purified water for granulation for 15 minutes;
[0063] (3) Dry the prepared granules in an oven at 40°C for 20 minutes, and control the moisture content of the granules at 1%;
[0064] (4) Add the dried particles into the mixer, set the speed at 60rpm, and mix for 10min;
[0065] (5) Add the lubricant into the mixer, continue mixing for 2-5 minutes, and take out the mixed materials for later use;
[0066] (6) Prepare the blank core material and purified water into a suspension, and the blank core material accounts for 25-35%;
[0067] (7) Spray the prepared suspension into the centrifugal granulator, set the rotation speed to 200rpm, set the spray speed to 30g / min, and set the drying tempera...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com