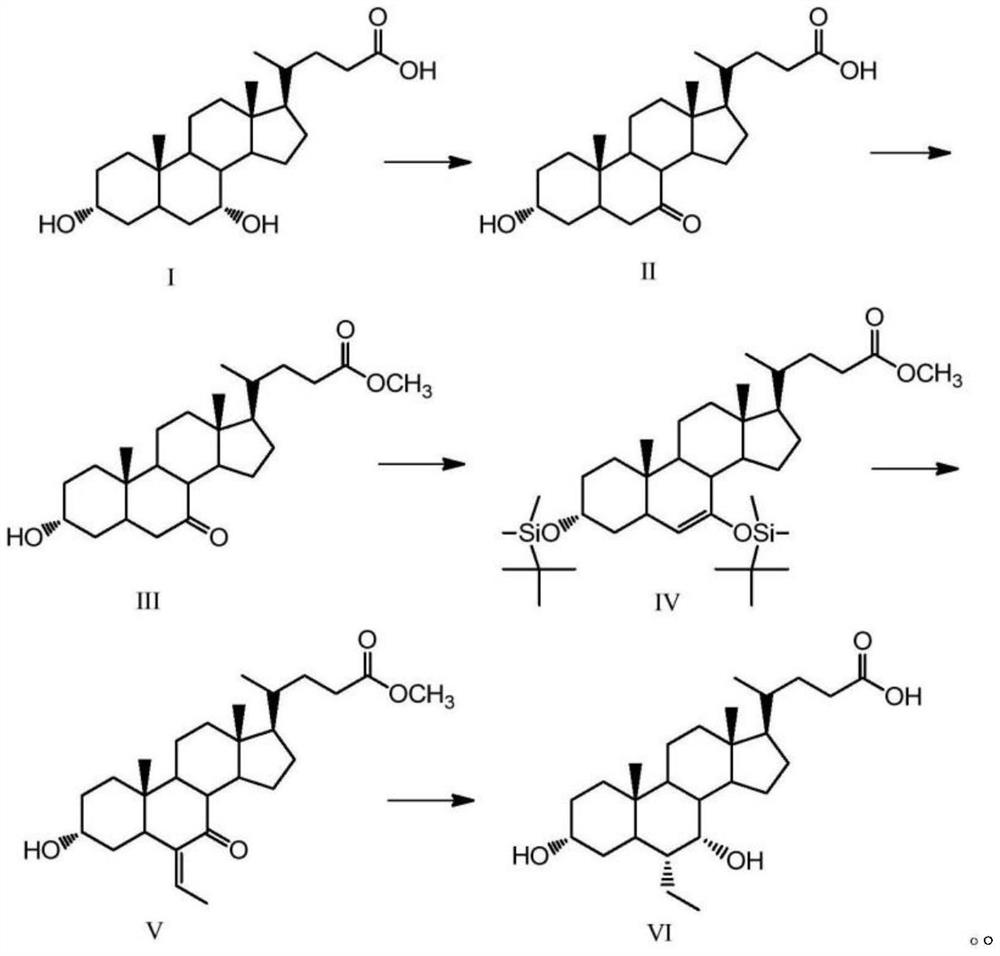
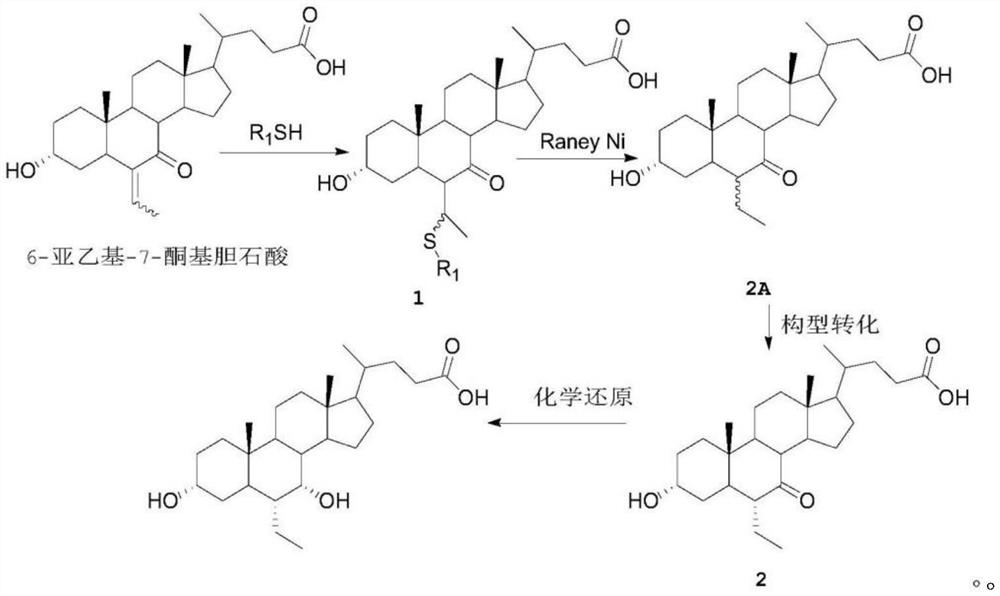
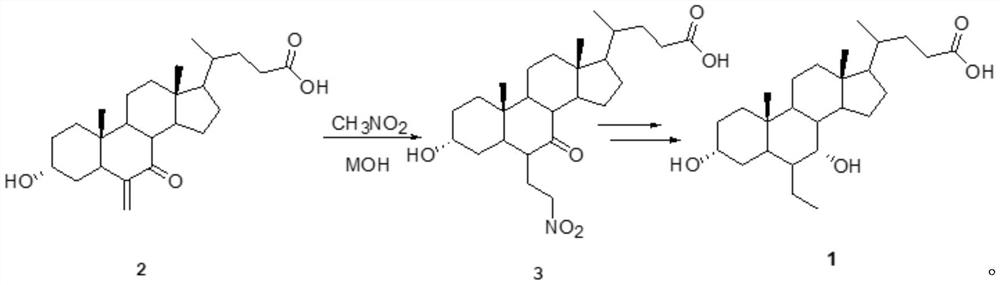
A kind of preparation method of obeticholic acid
A technology of obeticholic acid and ketocholic acid, which is applied in the fields of synthetic chemistry and biomedicine, can solve the problems of long synthetic routes and complicated steps, achieve the effects of low toxicity and hazard, facilitate scale-up production, and avoid production conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] (1) In a 1L reaction flask, put 40.2g (0.1mol) of 6-methylene-7-ketocholelithic acid, 400ml of dimethyl sulfoxide, and 40ml of nitromethane, and add anhydrous potassium hydroxide powder while stirring 18g (0.32mol), heat up, react at 80°C, TLC tracking, stop the reaction when the raw materials disappear, cool down to 20°C, add 500ml of water, continue to cool down to 0°C-5°C, and then add sodium dihydrogen phosphate until The pH value of the solution was below 6, and continued to stir overnight, and a white powdery solid was precipitated, filtered, fully washed with purified water, and dried to obtain 6-α-(2-nitroethyl)-7-ketocholelithic acid (compound 3 ), which was purified by recrystallization from 95% ethanol.
[0055] (2) Add compound 3 after purification to a 1L three-necked flask, add 400ml of methanol, 100 microliters of acetyl chloride, heat up and reflux for 1 hour, follow the completion of the reaction by TLC, distill off methanol and by-product water, and th...
Embodiment 2
[0058] (1) In a 1L reaction flask, put 40.2g (0.1mol) of 6-methylene-7-ketocholelithic acid and 400ml of nitromethane into it, add 18g (0.32mol) of anhydrous potassium hydroxide powder under stirring, and heat up , react at 100°C, TLC tracking, stop the reaction when the raw materials disappear, evaporate most of the nitromethane, then cool down to 20°C, add 500ml of water, continue to cool down to 0-5°C, and then add sodium dihydrogen phosphate , adjust the pH value of the solution to below 6, continue to stir overnight, white powdery solids are precipitated, filtered, fully washed with purified water, and dried to obtain 6-α-(2-nitroethyl)-7-ketocholelithic acid ( Compound 3), which was purified by recrystallization from 95% ethanol.
[0059] (2) Add 3 after purification to a 1L three-necked flask, add 200ml of dimethylformamide, 40ml of water, 42g of sodium bicarbonate, 39g of sodium sulfide, 70g of sodium hydrosulfite (sodium dithionite), and then heat up to 80-100°C The ...
Embodiment 3
[0062] (1) In a 1L reaction flask, put 40.2g (0.1mol) of 6-methylene-7-ketocholelithic acid and 400ml of nitromethane into it, add 18g (0.32mol) of anhydrous potassium hydroxide powder under stirring, and heat up , react at 90°C, TLC tracking, stop the reaction when the raw materials disappear, evaporate most of the nitromethane, then cool down to 20°C, add 500ml of water, continue to cool down to 0-5°C, and then add sodium dihydrogen phosphate , adjust the pH value of the solution to below 6, continue to stir overnight, white powdery solids are precipitated, filtered, fully washed with purified water, dried to obtain 6-α-(2-nitroethyl)-7-ketocholelithic acid ( Compound 3), which was purified by recrystallization from 95% ethanol.
[0063] (2) Compound 3 after purification was added to a 1L stainless steel autoclave, 400ml of methanol, 100ml of water, 15g of potassium dihydrogen phosphate, 4g of 5% silica-loaded platinum dioxide catalyst were added, vacuum pumped, hydrogen rep...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com