A gene medicine for the treatment of severe hypertriglyceridemia
A technology of gene medicine and gene expression, applied in drug combination, gene therapy, pharmaceutical formula, etc., can solve the problem of low immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
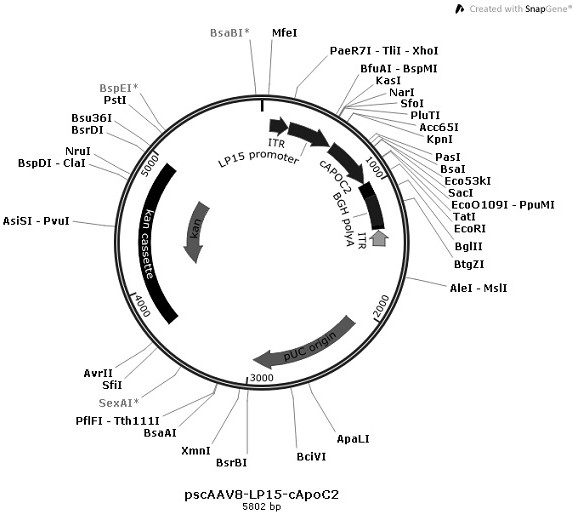
[0072] Example 1 Plasmid vector construction
[0073] In order to construct the pscAAV8-LP15-ApoC2 plasmid required for packaging recombinant AAV virus, we first use the pscAAV8-LP15-EGFP vector kept by the company [the ITR sequence on one side of the vector is trs (terminal resolution site) in the ITR of AAV2 deleted and the mutant sequence of D sequence, named ΔITR, as shown in SEQ ID No.7] as the basis, select the liver-specific high-efficiency expression promoter to regulate the expression of ApoC2 gene, optimize the sequence of ApoC2 coding region, and introduce in the optimized sequence Short introns improve gene transcription efficiency. Introducing four completely complementary target sequences of miR-142-3p in the 3'UTR region, reduced the expression of ApoC2 gene in antigen-presenting cells, and weakened the immune response caused by the overexpression of ApoC2. A short liver-specific enhancer was inserted at the 3' end of the expression frame to enhance the transcr...
Embodiment 2
[0078] Example 2 Preparation and assay of recombinant AAV virus
[0079] Three different packaging systems were used to package scAAV8-LP15-ApoC2 recombinant virus respectively.
[0080] Packaging system 1 Three-plasmid co-transfection HEK293 packaging system
[0081] References (Xiao X, et al . J Virol. 1998;72(3):2224-2232.), the three-plasmid packaging system was used to package the recombinant AAV virus, and the cesium chloride density gradient centrifugation method was used to separate, purify and package the AAV virus. Briefly, AAV vector plasmid (pscAAV8-LP15-ApoC2), helper plasmid (pHelper) and AAV Rep and Cap protein expression plasmid (pAAV-R2C8) were mixed at a molar ratio of 1:1:1, followed by the calcium phosphate method HEK293 cells were transfected, and 48 hours after transfection, the cells and culture supernatant were harvested, and the recombinant AAV virus was isolated and purified by cesium chloride density gradient centrifugation. Packaged and purified ...
Embodiment 3
[0091] Example 3 ApoC2 - / - Establishment of the hamster model
[0092] The ApoC2 knockout hamster model was provided by Mr. Liu Guoqing from Peking University Health Science Center. Patent name: Kit and method for constructing ApoC2 gene knockout hamsters. Patent application number: 201811179674.7.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com