Application of epicannabidiol hydrate in preparing drugs for preventing and/or curing brain injuries and pharmaceutical composition comprising epicannabidiol hydrate
A technology of epi-cannabidiol hydrate and brain injury, applied in the field of natural medicine and drug treatment, can solve the problem that the treatment effect of brain injury disease has not been reported, and can reduce the volume of cerebral infarction, reduce the volume of cerebral infarction, and reduce brain injury. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Determination of cellular anti-inflammatory pharmacological activity:
[0043] (1) Cytotoxicity assay:
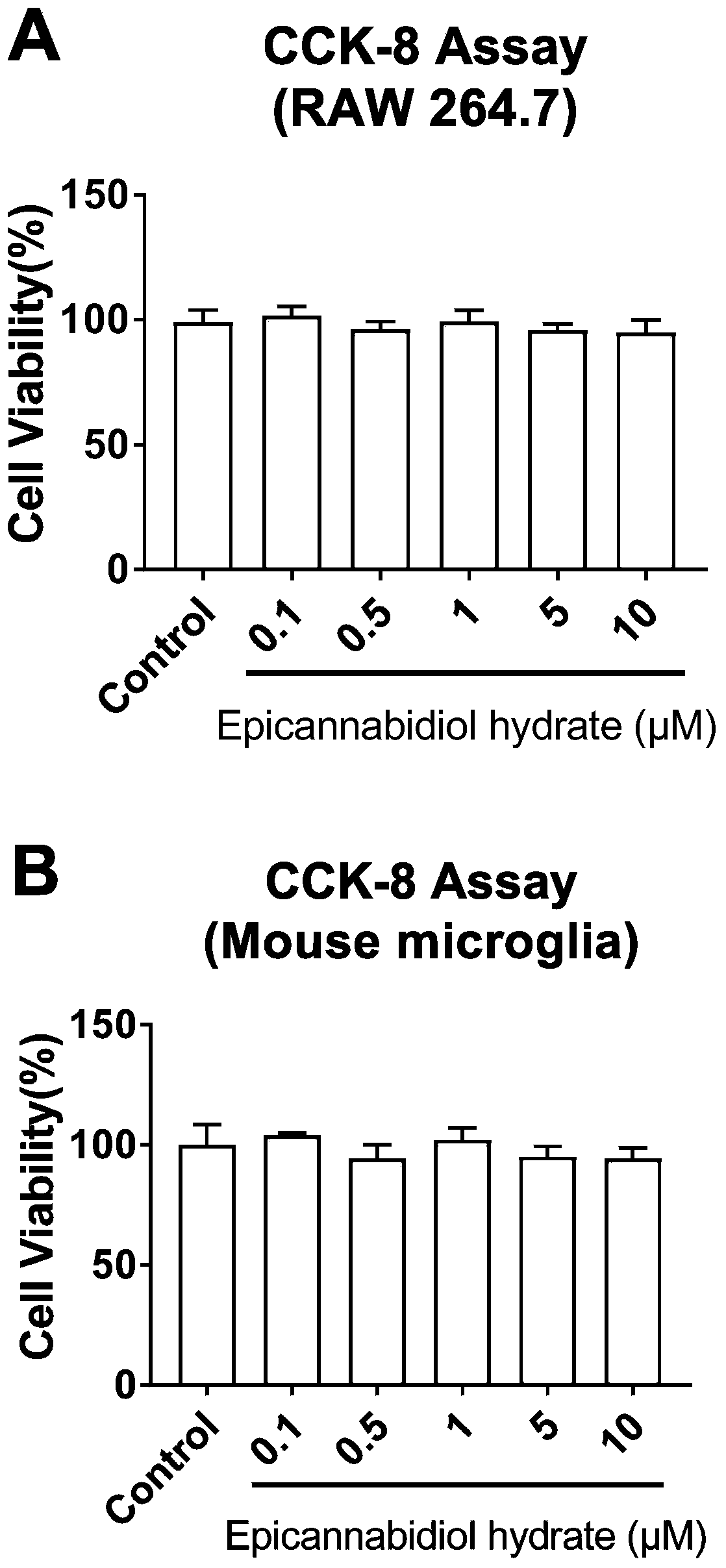
[0044] Dissolve epicannabidiol hydrate in dimethyl sulfoxide (DMSO) to prepare a solution with a concentration of 0.1, 1, 2, 5, 10, 20, and 50 μM, and add 100 μL 5000 to each well of a 96-well cell culture plate RAW264.7 cells or primary mouse microglial cells were added with 10 μL epicannabidiol hydrate solution of each concentration for 24 hours, and then 10 μL CCK-8 solution was added to each well, and the culture was continued for 2 hours, and measured at 450 nm Absorbance.
[0045] Test results such as figure 1 A and figure 1 As shown in B, where * P** P<0.01, vs Control, (Graphpad6.0, One-way Anova). It can be seen from the figure that epicannabidiol hydrate has no significant toxicity to RAW264.7 cells or primary mouse microglia at a concentration of 10 μM or below.
[0046] (2) Determination of cells releasing inflammatory factor proteins:
[0047] RAW...
Embodiment 2
[0069] Improvement effect of epicannabidiol hydrate on ischemic stroke
[0070] (1) Experimental grouping
[0071] Mice were randomly divided into 4 groups, 8 in each group, respectively sham operation group, ischemia-reperfusion model group (model group), compound treatment low-dose group (low-dose group, 1mg / kg) and compound treatment high-dose group (high dose group, 10mg / kg).
[0072] (2) Establishment of mouse transient middle cerebral artery occlusion model (tMCAO)
[0073] Place the mouse in an anesthesia induction box, give 2-2.5% isoflurane to induce anesthesia, take it out after the righting reflex disappears, put on a breathing mask, give 1-1.5% isoflurane to maintain anesthesia, and use Doppler After measuring the blood flow of the right brain with a blood flow meter, fix the mouse in the supine position, prepare the skin on the neck, disinfect with iodine, expose the right common carotid artery, external carotid artery and internal carotid artery, hang the threa...
Embodiment 3
[0091] Ameliorative effect of epicannabidiol hydrate on lipopolysaccharide-induced neuroinflammation in mice
[0092] (1) Experimental grouping and dosing regimen
[0093] The mice were randomly divided into 4 groups, 8 in each group, which were blank control group, model group, low-dose compound treatment group (low-dose group) and high-dose compound treatment group (high-dose group). 48h and 24h before modeling, epicannabidiol hydrate DMSO solution (1mg / kg, 10mg / kg) was injected into the tail vein, and the administration volume was 4mL / kg. The blank control group and the modeling group were injected with the same amount of DMSO .
[0094] (1) Establishment of a mouse neuroinflammation model induced by lipopolysaccharide
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com