Quaternary ammonium salt surfactant, preparation method and applications thereof
A surfactant and quaternary ammonium salt technology, which is applied in the field of high-efficiency and low-consumption quaternary ammonium salt surfactants, can solve the problems of low reactivity, strong corrosion, and harsh reaction conditions of polyols
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 2
[0095] The preparation of embodiment 1 dimer quaternary ammonium salt surfactant
[0096] The preparation method of dimer quaternary ammonium salt surfactant is as follows:
[0097]
[0098] The specific method is:
[0099] (1) Synthesis steps of the intermediate product diamide: add 14.1 g (160.0 mmol) of N,N-dimethylethylenediamine to 3.2 g (20.0 mmol) of DL-dimethyl malate, and reflux at 106 ° C for 3 h . After the reaction, use a rotary evaporator to remove excess N,N-dimethylethylenediamine to obtain an intermediate with a yield of 99%.
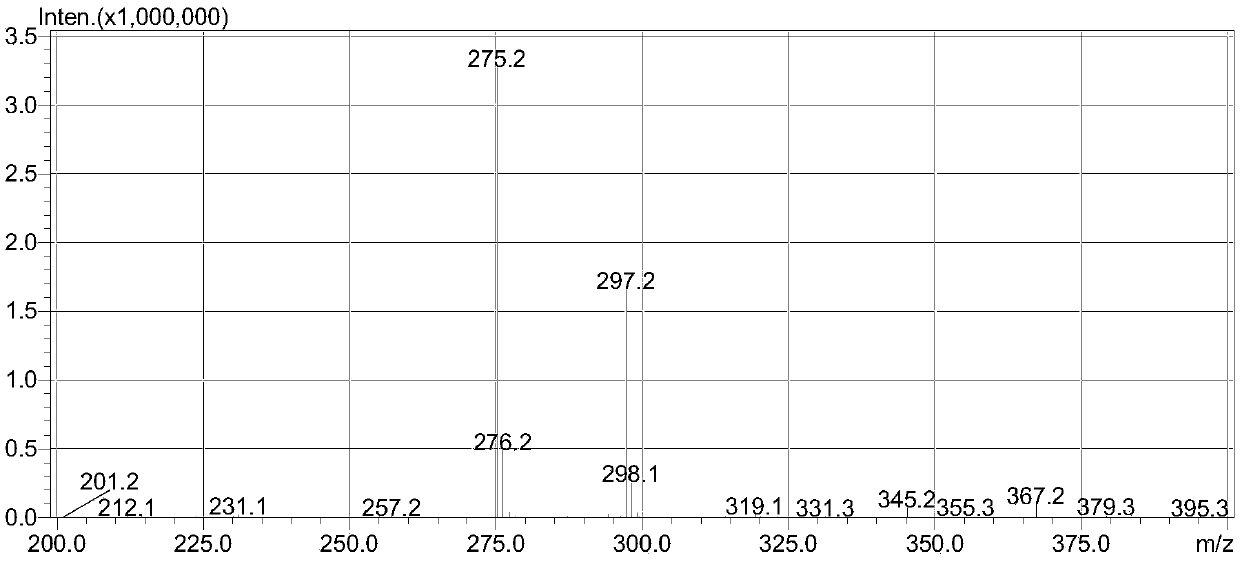
[0100] The intermediate was characterized by ESI-MS: 275.2 (M+H), 297.2 (M+Na).
[0101] 1 H NMR (CDCl 3 , 400MHz): δ=2.24 (single peak, 12H, -N(CH 3 ) 2 ), 2.44 (multiplet, 4H, -NH-CH 2 -CH 2 -N(CH 3 ) 2 ), 2.51, 2.82 (multiplet, 2H, -NH-CO-CH 2 -CH(OH)-CO-NH-), 3.25 (multiplet, 4H, -NH-CH 2 -CH 2 -N(CH 3 ) 2 ), 4.42 (single peak, 1H, -CH-OH), 5.95 (single peak, 1H, -CH-OH), 7.04, 7.49 (single peak, 2H, -NH-CO-CH 2 -...
Embodiment 2 3
[0106] The preparation of embodiment 2 trimerized quaternary ammonium salt surfactants
[0107] The preparation method of trimerized quaternary ammonium salt surfactant is as follows:
[0108]
[0109] The specific method is:
[0110] (1) Synthesis of intermediate tripolyamide: 10.6 g (120.0 mmol) of N,N-dimethylethylenediamine was added to 2.3 g (10.0 mmol) of trimethyl citrate, and refluxed at 106° C. for 3 h. After the reaction, use a rotary evaporator to remove excess N,N-dimethylethylenediamine to obtain an intermediate with a yield of 99%.
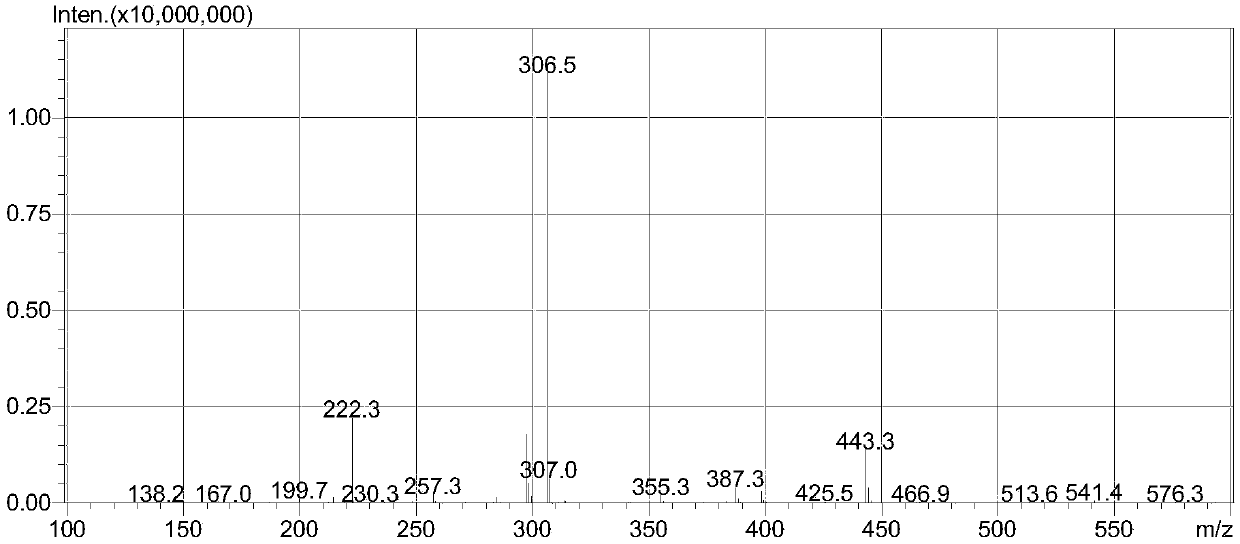
[0111] The intermediate was characterized by ESI-MS: 403.2 (M+H), 425.3 (M+Na).
[0112] 1 H NMR (CDCl 3 , 400MHz): δ=2.24 (single peak, 18H, -N(CH 3 ) 2 ), 2.41 (multiplet, 6H, -NH-CH 2 -CH 2 -N(CH 3 ) 2 ), 2.60-2.73 (multiple peaks, 4H, -NH-CO-CH 2 -CH(OH)-), 3.32 (multiplet, 6H, -NH-CH 2 -CH 2 -N(CH 3 ) 2 ), 5.20 (unimodal, 1H, -CH-OH), 7.10, 7.47 (unimodal, 3H, -NH-CH 2 -CH 2 -N(CH 3 ) 2 ).
[0113] (2) Pre...
Embodiment 3
[0116] Embodiment 3 linking group contains the preparation of the dimeric surfactant of aromatic ring
[0117] The preparation method of the dimeric surfactant that linking group contains aromatic ring is as follows:
[0118]
[0119] The specific method is:
[0120] (1) Synthesis of intermediate dimethyl ester: 2.7 g (10.0 mmol) of azobenzene-3,3'-dicarboxylic acid was added to 50 mL of methanol, and a catalytic amount of concentrated sulfuric acid was added to reflux for 3 h. After the reaction, the solvent was removed by a rotary evaporator to obtain the intermediate dimethyl ester with a yield of 99%.
[0121] (2) Synthesis of intermediate diamine: 3.5 g (40.0 mmol) of N,N-dimethylethylenediamine was added to 3.0 g (10.0 mmol) of intermediate dimethyl ester, and refluxed at 106° C. for 3 h. After the reaction, use a rotary evaporator to remove excess N,N-dimethylethylenediamine to obtain an intermediate with a yield of 99%.
[0122] (3) The preparation of the dipolym...
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface tension | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com