A composition for adjuvant treatment of chronic bleeding and its application
A technology of adjuvant therapy and composition, applied in the field of cell biology, molecular biology and drug research and development, can solve the problems of no particularly effective treatment method, high cost, short-term effect, etc., to avoid infection or immune rejection, and easy to use , long-lasting effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
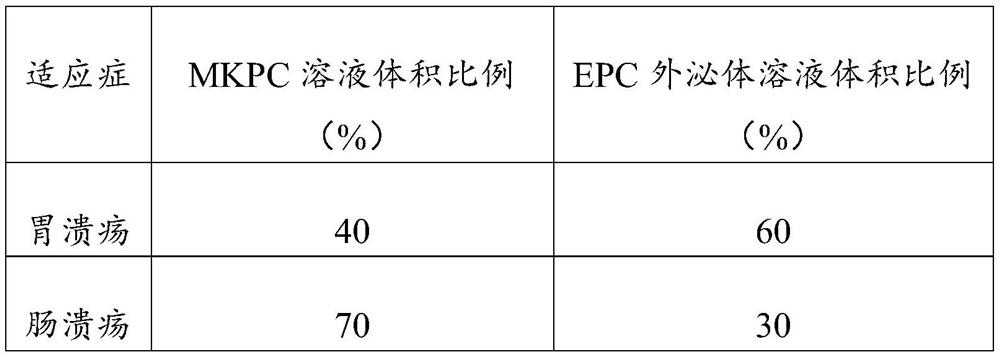
[0034] According to different indications, MKPC and EPC exosomes are mixed in different proportions to obtain pharmaceutical preparations for adjuvant treatment of chronic bleeding. MKPC with 1*10 5 The MKPC solution obtained by dissolving 1 cell in 1 mL of normal saline is 1 unit of MKPC, and the EPC exosomes are used per 1*10 5 The EPC solution obtained by dissolving the exosomes (0.1-0.18 μg) extracted by EPC in 1 mL of normal saline is 1 unit of EPC exosomes. For different chronic bleeding conditions, different proportions of MKPC solution and EPC should be combined. The solution is the drug preparation for adjuvant therapy in a total volume of 4 mL. The details are shown in Table 1.
[0035] Table 1 MKPC and EPC exosome content ratio (calculated by volume) in the composition of different indications
[0036]
Embodiment 2
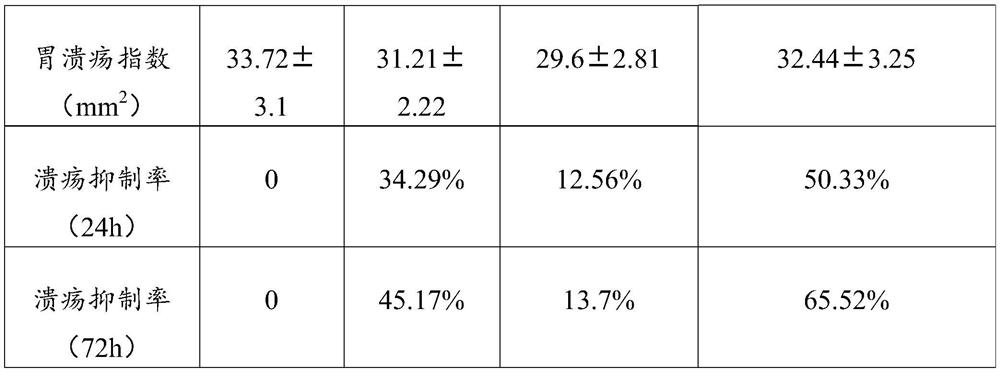
[0038] The gastric ulcer animal experiment of the pharmaceutical preparation of the above-mentioned Example 1 was taken, and the pharmaceutical preparation contained a volume ratio of 40% MKPC solution and a volume ratio of 60% EPC exosome solution.
[0039] Animal model of gastric mucosal injury: 24 healthy Wistar rats were randomly divided into control group, MKPC group, EPC exosome group and MKPC+EPC exosome group according to body weight.
[0040] The experimental acetic acid-induced gastric ulcer model in rats was prepared by acetic acid smearing method. The animal model was treated by intravenous infusion. The control group was infused with 4 mL of normal saline, and the MKPC group was infused with an equal amount of normal saline solution containing MKPC (that is, containing 4*10 5 normal saline solution of MKPC), the EPC exosome group was infused with the same amount of normal saline solution containing EPC exosome (that is, containing cultured 4*10 5 The normal sali...
Embodiment 3
[0047] The intestinal ulcer animal experiment of the pharmaceutical preparation of the above-mentioned Example 1 was taken, and the pharmaceutical preparation contained a volume ratio of 70% MKPC solution and a volume ratio of 30% EPC exosome solution.
[0048] Ulcerative colitis animal model: 32 healthy Wistar rats were randomly divided into control group, MKPC group, EPC exosome group and MKPC+EPC exosome group according to body weight.
[0049] An experimental rat intestinal ulcer model was prepared by using 50% ethanol solution containing 150mg / kg trinitrobenzenesulfonic acid (TNBS). The animal model is treated by intravenous infusion, the treatment period is 7 days, by PGE 2 The detection identifies the outcome of the treatment. The control group was infused with 4 mL of normal saline, and the MKPC group was infused with an equal amount of normal saline solution containing MKPC (that is, containing 4*10 5 normal saline solution of MKPC), the EPC exosome group was infuse...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com