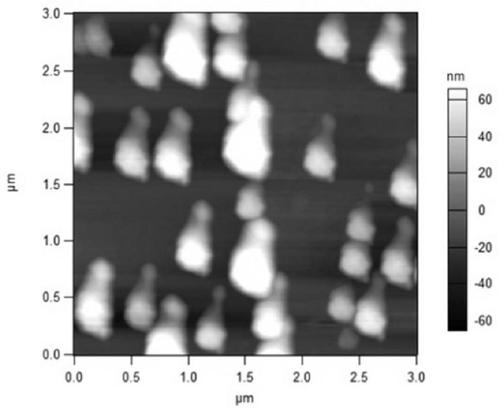
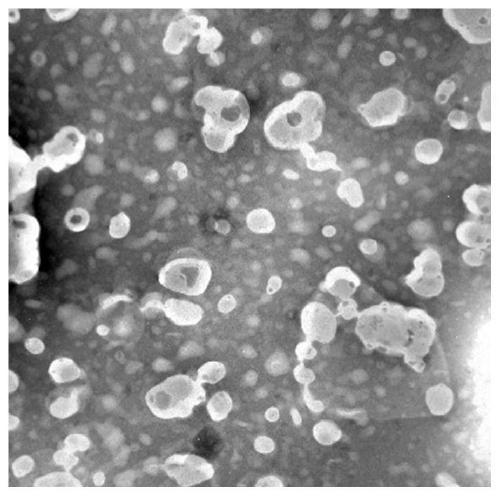
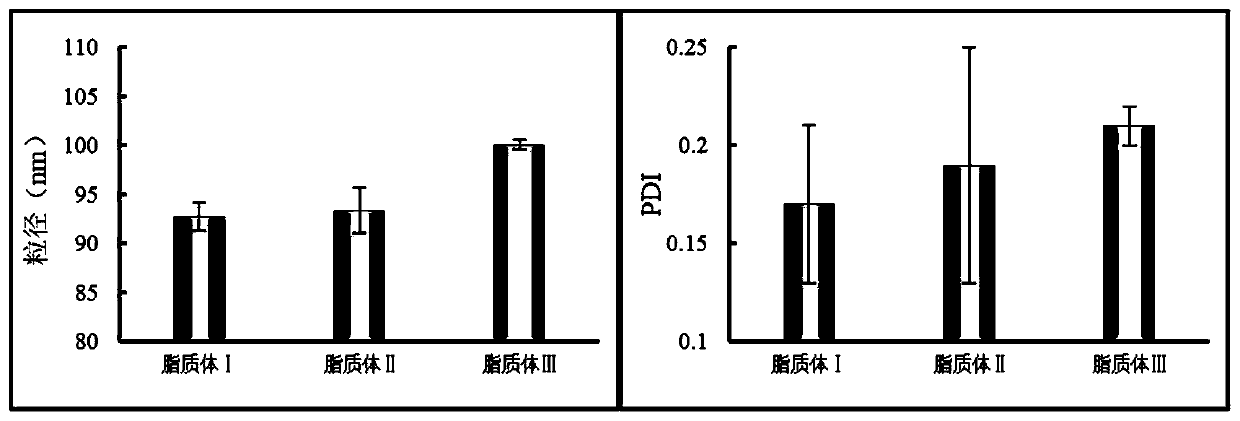
Baicalin lipidosome and application thereof
A technology of baicalin and baicalin, which is applied in the field of pharmaceutical preparations, can solve the problems of accelerated liposome degradation, easy precipitation, liposome instability, etc., and achieve the effects of increasing elasticity, uniform particle size, and improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The preparation of embodiment 1 baicalin liposome I of the present invention
[0048] Precisely weigh 200mg of phospholipids, 20mg of cholesterol and 20mg of ceramides and place them in a 500mL round bottom flask at the same time, add appropriate amount of ethanol and ultrasonically assist to dissolve them completely. The ethanol was removed by rotary evaporation in a water bath at 50°C, and a light yellow film was formed on the inner wall of the flask. Add 250ml-1000ml of 250mM ammonium sulfate buffer solution, hydrate at 40°C for 20 minutes, and dissolve with the aid of ultrasound; then filter, homogenize the filtrate through a high-pressure homogenizer for 5-15 times, and then use HBS for 24h dialysis, baicalin aqueous solution (5mg / ml, pH6.5-7.5) 1ml, stirred in a 40-degree water bath for 20 minutes to obtain baicalin liposome I.
Embodiment 2
[0049] The preparation of embodiment 2 baicalin liposome II of the present invention
[0050] Precisely weigh 200 mg of phospholipids, 16 mg of cholesterol, 2 mg of triolein, 2 mg of cholesterol oleate, and 20 mg of ceramide into a 500 mL round bottom flask at the same time, and add an appropriate amount of ethanol to assist in ultrasonication to dissolve them completely. The ethanol was removed by rotary evaporation in a water bath at 50°C, and a yellow film was formed on the inner wall of the flask. Add 250ml-1000ml of 250mM ammonium sulfate buffer solution, hydrate in a water bath at 40°C for 20 minutes, and dissolve with the aid of ultrasound; then filter, homogenize the filtrate through a high-pressure homogenizer for 5-15 times, and then use HBS for dialysis for 24 hours, baicalin Take 1ml of aqueous solution (10mg / ml, pH6.5-7.5), stir in a 40-degree water bath for 20 minutes to obtain baicalin liposome II.
Embodiment 3
[0051] Embodiment 3 The preparation of baicalin liposome III of the present invention
[0052] Accurately weigh 200 mg of phospholipids, 14 mg of cholesterol, 2 mg of glyceryl trioleate, 2 mg of medium chain triglycerides, 2 mg of macrogol glycerol oleate and 20 mg of ceramide in a 500 mL round-bottomed flask at the same time. Aids in complete dissolution. The ethanol was removed by rotary evaporation in a water bath at 50°C, and a yellow film was formed on the inner wall of the flask. Add 250ml-1000ml of 250mM ammonium sulfate buffer solution, hydrate in a water bath at 40°C for 20 minutes, and dissolve with the aid of ultrasound; then filter, homogenize the filtrate through a high-pressure homogenizer for 5-15 times, and then use HBS for dialysis for 24 hours, baicalin Take 1ml of aqueous solution (20mg / ml, pH6.5-7.5), and stir in a water bath at 40°C for 20 minutes to obtain baicalin liposome III.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com