Bis(N-phenyl)-3-carbazole substituted phenanthroimidazole compound, preparation method thereof and application thereof as electroluminescent device
A technology of phenanthroimidazole and compounds, which is applied in the field of organic light-emitting materials and optoelectronic devices, can solve the problems of unbalanced carrier transport and insufficient purity of luminous color, achieve carrier injection and transport balance, and improve fluorescence quantum Efficiency, the effect of achieving high fluorescence quantum efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054]The bis-N-phenyl-3-carbazole-substituted phenanthroimidazole compound has a molecular structure as shown in formula (I).
[0055] The preparation method of the phenanthroimidazole compound substituted by two N-phenyl-3-carbazoles comprises the steps:
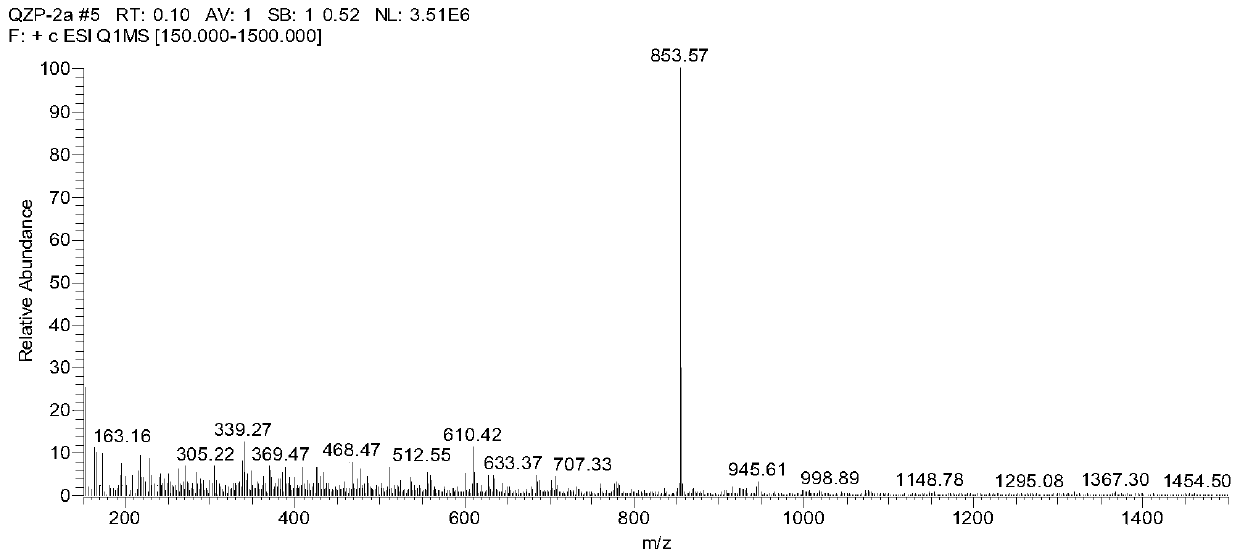
[0056] S1. Add 4-bromobenzaldehyde (1.86g, 10mmol), p-bromoaniline (1.70g, 10mmol), 9,10-phenanthrenequinone (2.08g, 10mmol), ammonium acetate (4.62g, 60mmol) into 100mL of two-necked flask, and added 60mL of glacial acetic acid to obtain a dark brown suspension. After the mixture was stirred at 120° C. for 2 hours, the color of the solution changed from dark brown to black, and the reaction mixture was stirred overnight (12 hours) at room temperature. The crude product was isolated by washing with methanol and filtration, then dried in vacuo. Using silica gel powder as stationary phase, petroleum ether and dichloromethane as eluent (petroleum ether: CH 2 Cl 2 , 1:2) to purify the product to obtain a white powder of bi...
Embodiment 2
[0064] Embodiment 2 performance test
[0065] Taking the double N-phenyl-3-carbazole modified phenanthroimidazole prepared in Example 1 as the test object, test its photophysical properties and other luminescent properties, the test results are as follows Figure 3 to Figure 8 shown.
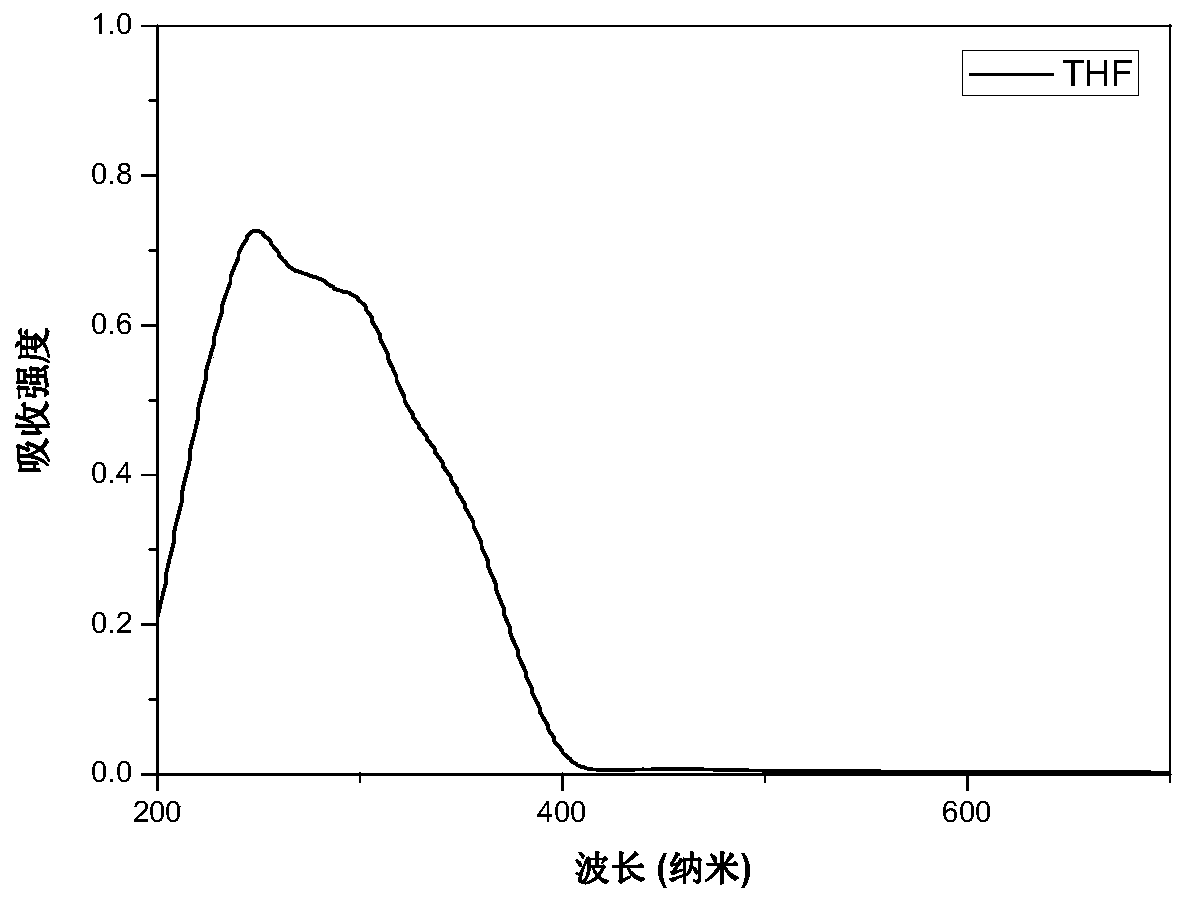
[0066] image 3 It is the normalized absorption spectrum of molecule M1 under tetrahydrofuran measured by Shimadzu UV-2700 ultraviolet-visible spectrophotometer. The results show that the molecule exhibits the maximum absorption intensity at 250nm, which is attributed to the π-π* transition of the benzene ring.
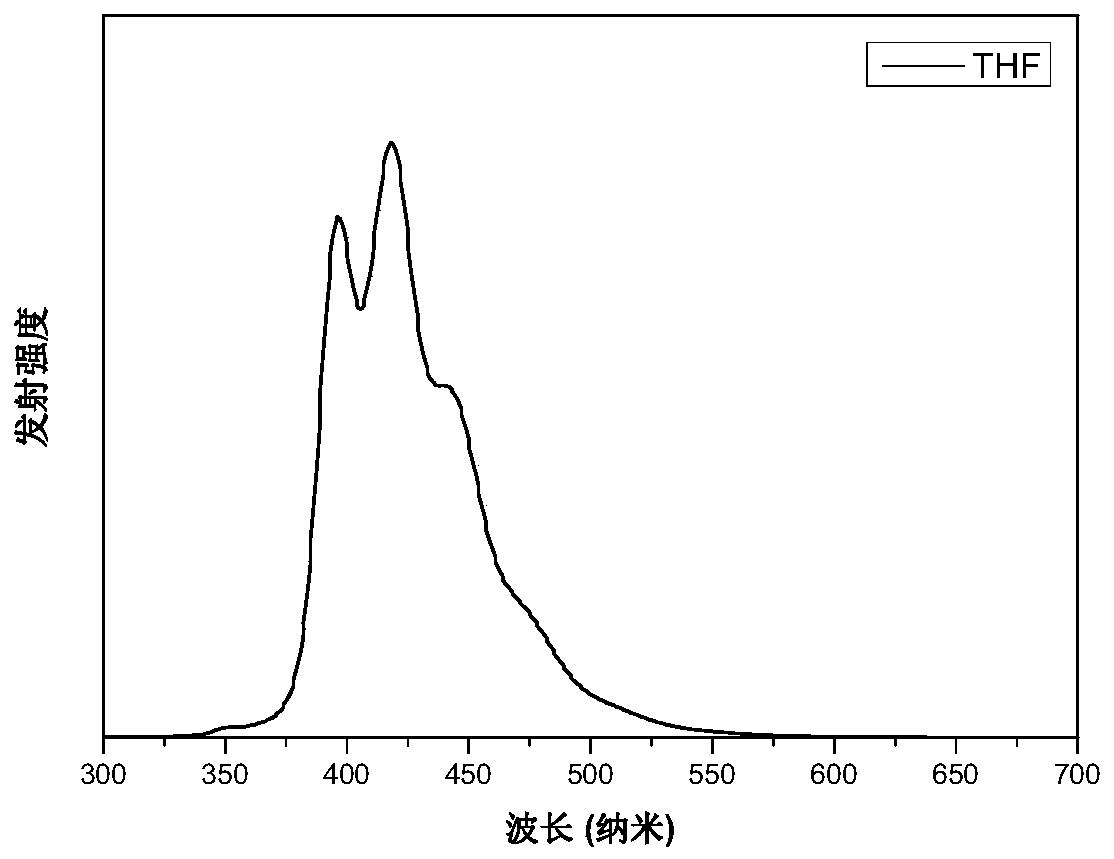
[0067] Figure 4 The fluorescence emission spectrum of the obtained molecule M1 was tested on an Edinburgh FLS980 at an excitation wavelength of 350 nm. The results show that the molecule emits fluorescence efficiently in tetrahydrofuran solution, and its fluorescence peak is located between 390-440nm, showing deep blue fluorescence emission.
[0068] Figure 5 It is the normalize...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com