Method for synthesizing 2-isopropylnaphthalene
A technology of isopropylnaphthalene and alkylation reagent, which is applied in the field of synthesizing 2-isopropylnaphthalene, can solve the problems of low conversion rate of raw materials and low selectivity of 2-isopropylnaphthalene, so as to improve catalytic efficiency, simplify The effect of precise control of process flow, reaction temperature and time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
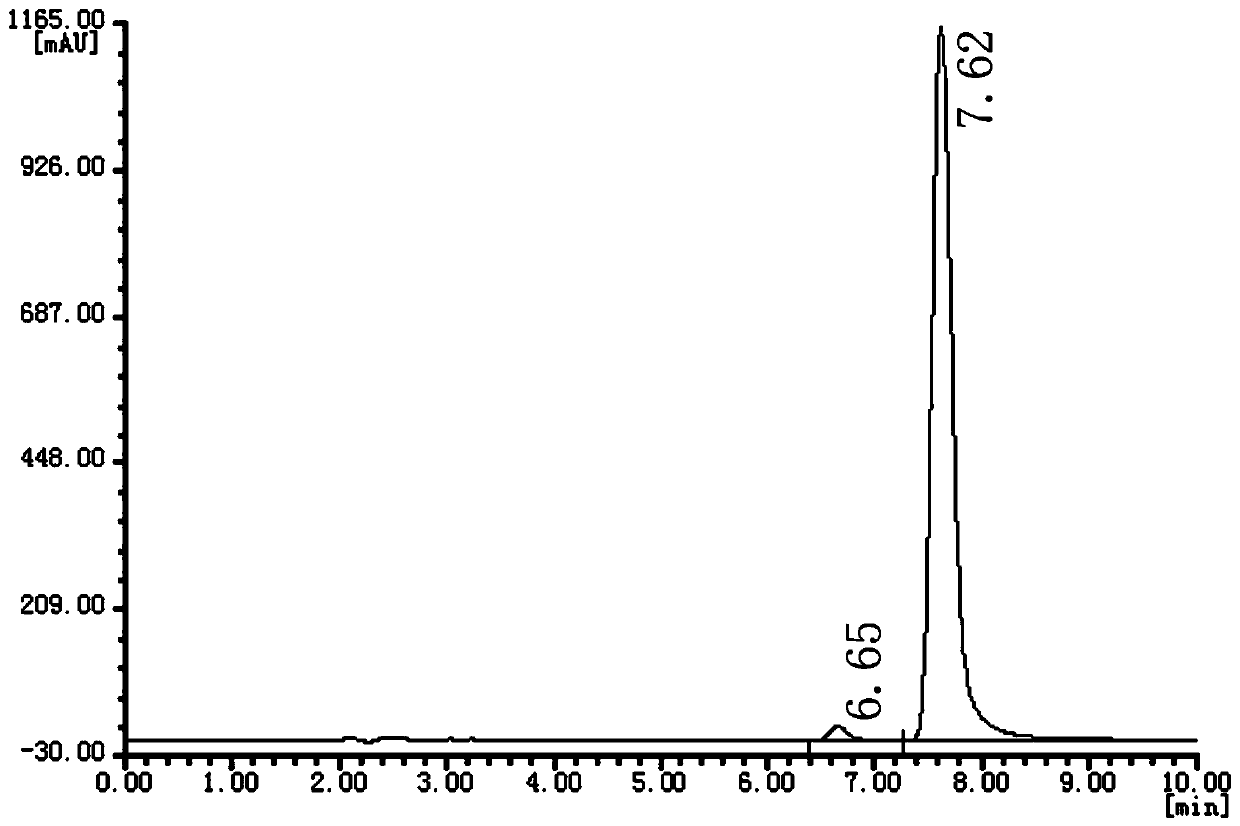
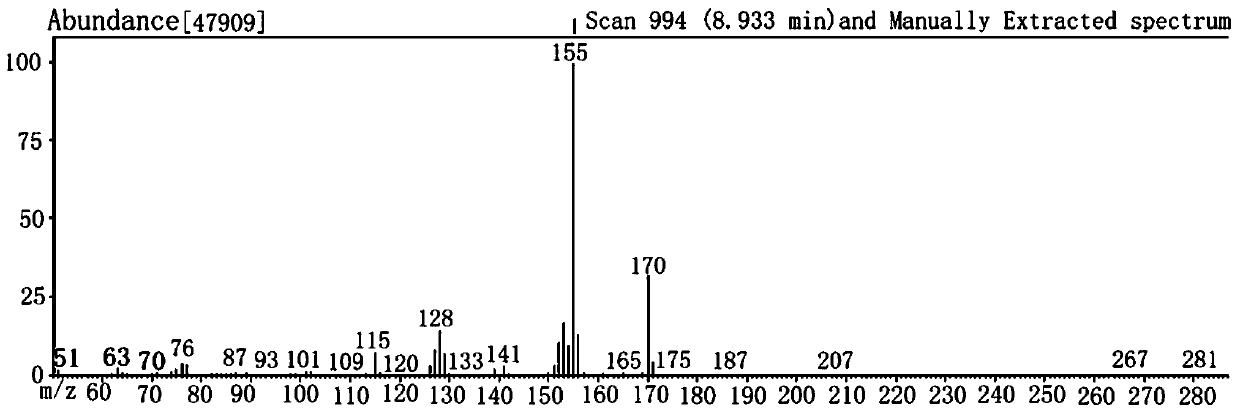
Examples
Embodiment 1
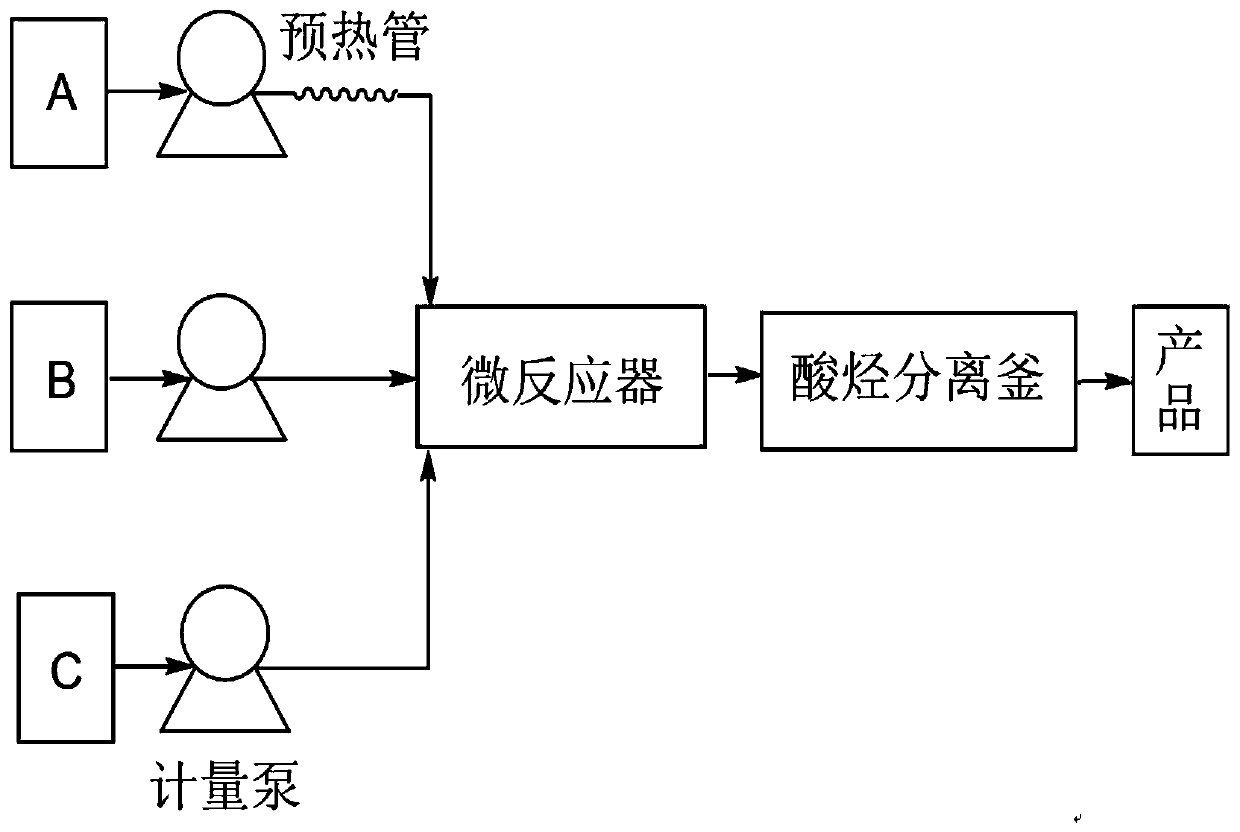
[0042] Such as figure 1 As shown, the device for synthesizing styrenated phenol in this embodiment includes preheating pipe, metering pump, microreactor (continuous flow microchannel reactor), and the microchannel cross-sectional equivalent diameter of microreactor in this embodiment is 0.5mm , the microchannel tube length is 100m.
[0043] The steps of synthesizing styrenated phenol in the present embodiment are as follows:
[0044] (1) Naphthalene is heated to 90 DEG C and kept warm by a preheater as fluid A; 2-bromopropane is used as fluid B at normal temperature; triethylamine heptachlorodialuminate ionic liquid is used as fluid C at normal temperature; wherein, The molar ratio of naphthalene: 2-bromopropane: chloroaluminate ionic liquid is 1: 1.04: 0.6;
[0045] The volume flow rate of naphthalene is controlled by a metering pump to be 2.5ml / min, the volume flow rate of 2-bromopropane is 2.0ml / min and the volume flow rate of chloroaluminate ionic liquid catalyst is 3ml / ...
Embodiment 2
[0050] Such as figure 1 As shown, the device for synthesizing styrenated phenol in this embodiment includes preheating pipe, metering pump, microreactor (continuous flow microchannel reactor), and the microchannel cross-sectional equivalent diameter of microreactor in this embodiment is 0.6mm , the microchannel tube length is 120m.
[0051] The steps of synthesizing styrenated phenol in the present embodiment are as follows:
[0052] (1) Naphthalene is heated to 95 DEG C and kept warm by a preheater as fluid A; 2-bromopropane is used as fluid B at normal temperature; triethylamine heptachlorodialuminate ionic liquid is used as fluid C at normal temperature; wherein, The molar ratio of naphthalene: 2-bromopropane: chloroaluminate ionic liquid is 1: 1.04: 1.2;
[0053] The volume flow rate of naphthalene is 3ml / min, the volume flow rate of 2-bromopropane is 2.3ml / min, and the volume flow rate of chloroaluminate ionic liquid catalyst is 6ml / min through the metering pump, and th...
Embodiment 3
[0056] Such as figure 1 As shown, the device for synthesizing styrenated phenol in this embodiment includes preheating pipe, metering pump, microreactor (continuous flow microchannel reactor), and the microchannel cross-sectional equivalent diameter of microreactor in this embodiment is 0.8mm , the microchannel tube length is 150m.
[0057] The steps of synthesizing styrenated phenol in the present embodiment are as follows:
[0058] (1) Naphthalene is heated to 90 DEG C and kept warm by a preheater as fluid A; 2-bromopropane is used as fluid B at normal temperature; triethylamine heptachlorodialuminate ionic liquid is used as fluid C at normal temperature; wherein, The molar ratio of naphthalene: 2-bromopropane: chloroaluminate ionic liquid is 1: 1.04: 1.8;
[0059] The volume flow rate of naphthalene is controlled by a metering pump to be 3.5ml / min, the volume flow rate of 2-bromopropane is 2.7ml / min and the volume flow rate of chloroaluminate ionic liquid catalyst is 9ml / ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com