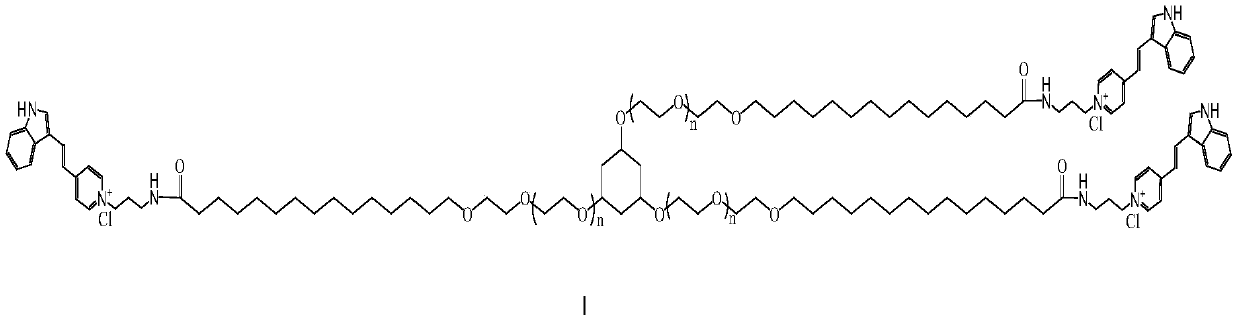
Novel cationic surfactant and preparation method thereof
A technology of surfactant and cation, which is applied in the field of preparation of indole quaternary ammonium salt, can solve the problems of poor biodegradability and water solubility, and reduce the foam of surfactant system, etc., and achieve simple steps, good emulsification and compounding performance Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Synthesis of Indole Quaternary Ammonium Salt of Monodisperse Pentadecyl Three-Armed Polyethylene Glycol Ether
[0030] The synthesis process contains three steps: (1) Synthesis of three-arm dodecapolyethylene glycol p-toluenesulfonate 2) Synthesis of pentadecanoic acid three-arm dodecapolyethylene glycol; (3) Pentadecyl three Synthesis of Indole Quaternary Ammonium Salts of Armed Polyethylene Glycol Ethers.
[0031] (1) Synthesis of three-arm polyethylene glycol p-toluenesulfonate
[0032] At 0°C, slowly add p-toluenesulfonyl chloride (10mmol) dropwise to a solution of three-arm dodecapolyethylene glycol (3mmol) and triethylamine (3mmol) in dichloromethane (30ml), and continue stirring at room temperature after the dropwise addition 12 hours. The reaction was quenched by saturated sodium bicarbonate (100 mL), and then separated. After the organic phase was concentrated under reduced pressure, the crude product was directly put into the next reaction.
[0033] (2) Syn...
Embodiment 2
[0038] Synthesis of Indole Quaternary Ammonium Salt of Monodisperse Pentadecyl Three-Armed Tetracospolyethylene Glycol Ether
[0039] The synthesis process contains three steps: (1) synthesis of three-arm tetracos-polyethylene glycol to DMF sulfonate; 2) synthesis of pentadecanoic acid three-arm tetracos-polyethylene glycol; (3) pentadecane Synthesis of indole quaternary ammonium salts of three-arm tetracospolyethylene glycol ethers.
[0040] (1) Synthesis of three-arm tetracos-polyethylene glycol p-toluenesulfonate
[0041] At 0°C, slowly add p-DMFsulfonyl chloride (10mmol) dropwise to a solution of three-arm tetracosethylene glycol (3mmol) and triethylamine (3mmol) in dichloromethane (30ml), and continue at room temperature after the addition is complete. Stir for 12 hours. The reaction was quenched by saturated sodium bicarbonate (100 mL), and then separated. After the organic phase was concentrated under reduced pressure, the crude product was directly put into the next ...
Embodiment 3
[0047] Synthesis of Indole Quaternary Ammonium Salt of Monodisperse Pentadecyl Three-Armed Fourty-Octacopolyethylene Glycol Ether
[0048] The synthesis process contains three steps: (1) synthesis of three-arm forty-octacopolyethylene glycol to DMF sulfonate; 2) synthesis of pentadecanoic acid three-arm forty-eight polyethylene glycol; Synthesis of indole quaternary ammonium salts of polyethylene glycol ethers.
[0049] (1) Synthesis of three-arm forty-octacopolyethylene glycol p-toluenesulfonate
[0050] At 0°C, slowly add p-toluenesulfonyl chloride (10mmol) dropwise to a solution of three-armed tetraoctaccopolyethylene glycol (3mmol) and triethylamine (3mmol) in dichloromethane (30ml), and continue stirring at room temperature for 12 Hour. The reaction was quenched by saturated sodium bicarbonate (100 mL), and then separated. After the organic phase was concentrated under reduced pressure, the crude product was directly put into the next reaction.
[0051] (2) Synthesis of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com