Alkenyl side-chain conjugated indacenodifuranyl polymer material as well as preparation method and application thereof
A polymer material, furan-based technology, used in semiconductor/solid-state device manufacturing, photovoltaic power generation, electrical components, etc., can solve the problem of reducing the degree of conjugation of IDT units, the contribution of π-conjugated systems of IDT units, and the light absorption capacity of polymers. The effect is not ideal and other problems, to achieve the effect of good photovoltaic performance, good photophysical performance and electrochemical performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
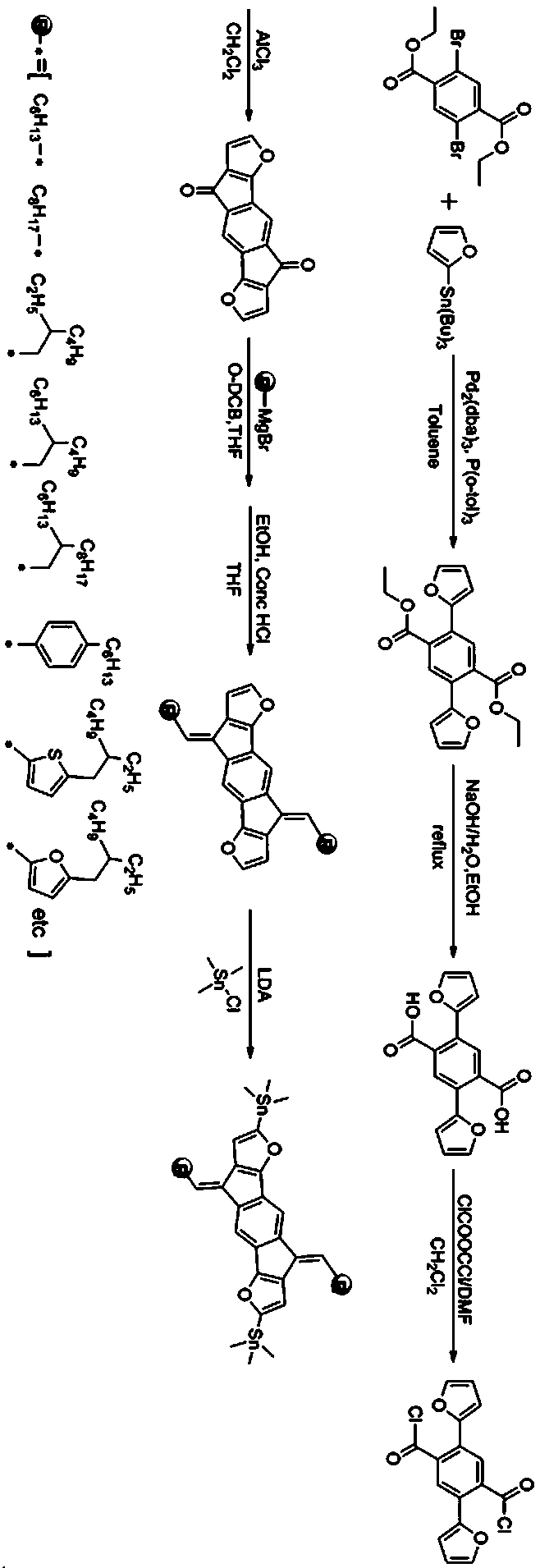
[0054] Synthesis of difuran-based polymer material (PIDF-TC6C8-BT) with conjugated alkenyl side chain
[0055] The synthetic route is as follows:
[0056]
[0057] (1) Synthesis of compound 2
[0058] With 2,5-dibromo-diethyl terephthalate (1.52g, 4.0 mmol), 2-tributyltinfuran (2.90g, 9.2mmol), three (dibenzylideneacetone) two palladium (0.037g, 0.04 mmol), tris(o-methylphenyl)phosphine (0.013 g, 0.044 mmol) were placed in a 100 mL single-necked flask, vacuumed and filled with nitrogen cyclically 2-3 times, and 50 mL of anhydrous toluene was added under the protection of nitrogen . Place the reaction flask in an oil bath and heat to 120 o C overnight (14~16h). After the mixture was cooled to room temperature, the mixed liquid was poured into deionized water, and the aqueous phase was extracted with 3×30 mL of dichloromethane. The organic phases were combined, washed with a saturated aqueous sodium chloride solution, the organic phases were collected and dried over anhydrous magne...
Embodiment 2
[0075] The specific process is the same as in Example 1, except that 1-bromo-2-hexyl-decane in step (5) is replaced by n-hexadecane.
[0076] Add IDF-T-C16 (0.20 mmol, 206.5 mg), 4,7-dibromo-2,1,3-bisfluorobenzothiadiazole (0.21 mmol, 60.3 mg), tetrakis (triphenylphosphine) palladium (11.56mg, 0.01mmol) is added to the N 2 Protect the device in a 25.0 mL single-necked flask. Then, evacuate and fill with nitrogen cyclically 3-4 times, and inject 10 mL of anhydrous toluene and 1 mL of anhydrous N,N-dimethylformamide with a syringe under the protection of nitrogen. After the addition, the mixture was evacuated again and filled with nitrogen. Then quickly heat to 120 o C reflux the reaction, and observe the change in the viscosity of the mixture. When the rotor speed in the reaction flask slows down and the mixture becomes viscous, use a syringe to inject 0.1 mL of 2-tributylstannane furan, and continue to stir for 2.0 h. Then use a syringe to inject 0.1 mL of p-bromofluorobenzene...
Embodiment 3
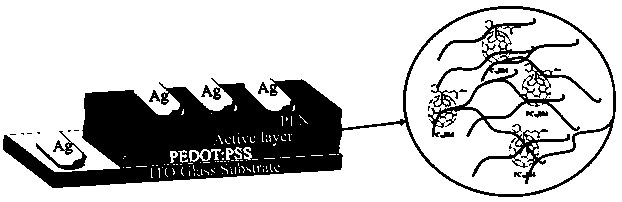
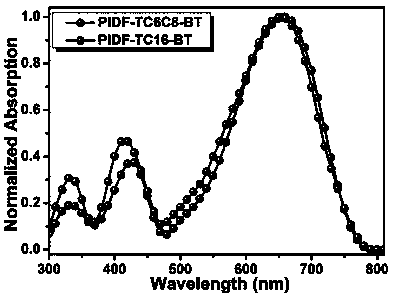
[0080] The alkenyl side chain conjugated difuran-based polymer materials (PIDF-TC6C8-BT and PIDF-TC16-BT) prepared in Example 1-2 were dissolved in chloroform to form 10 -4 mg / mL solution, and use a pipette to measure 60μL of chloroform solution spin-coated on pretreated quartz glass (15mm×15mm), air dry, test the UV-visible absorption spectrum of the polymer film, see details Figure 4 . According to formula E gap =1240 / λ onset It is calculated that the optical energy gaps of PIDF-TC6C8-BT and PIDF-TC16-BT under the spectrum are both 1.57eV, which are narrow band gap polymer materials.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com