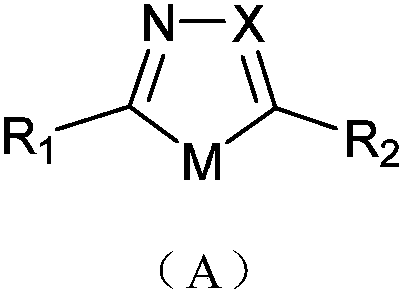
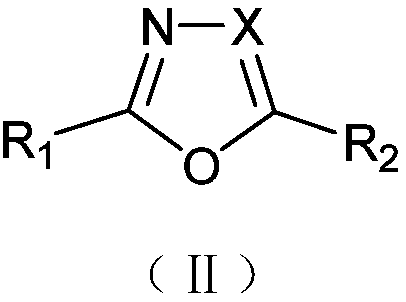
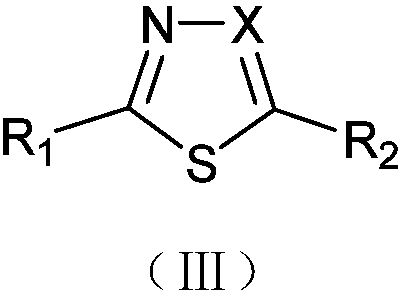
Solvent-free solid-phase synthesis method and application of microwave digestion of five-membered heterocyclic azoles containing oxygen, sulfur and nitrogen
The technology of a compound and a cyclizing agent, applied in the application field of the three series of compounds, can solve the problems such as few reports on the application of organic synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~8
[0062] 1. Embodiment 1~8 is the synthesis of oxygen-containing substituted five-membered heterocyclic azole compounds
Embodiment 1
[0063] The synthesis of embodiment 1,2-indolyl-5-phenyloxazole (Ⅱa1)
[0064]
[0065] Weigh 2.78g of N-(2-oxo-2-phenyl)ethyl-1H-indole-3-carboxamide and 1.7g of phosphorus pentoxide in the glove box and put them in an agate mortar in the glove box , mix evenly, grind finely, transfer to a 10mL reaction bottle in a microwave reactor, adjust the power of the microwave reactor to 560W, start the microwave reactor and heat up from room temperature to 230°C at a heating rate of 45°C / min, then react at a constant temperature for 30 Minutes, close the microwave reactor, stop the reaction, cool down to room temperature naturally, wash the mixture in the reaction flask with distilled water, dry, recrystallize from ethanol, and weigh 2.33g (IIa1) as a pale white solid, with a yield of 89.7%. mp:188~190℃.IRν(cm -1 ):3413,3154,2919,1625,1602,1443,1361,1249,1120,914,767,726; 1 H-NMR(400MHz,DMSO-d6):8.10–8.07(m,2H),7.98–7.95(m,2H),7.60–7.48(m,5H),7.25–7.17(m,2H); 13 C-NMR (125MHz, DM...
Embodiment 2
[0067] The synthesis of embodiment 2,2-indolyl-5 pyridyl oxazole (IIa2)
[0068]
[0069] Weigh 2.78g of N-(2-oxo-2-pyridyl)ethyl-1H-indole-3-carboxamide in the glove box, measure 1.5mL of phosphorus oxychloride, put into 10mL of microwave reactor to react In the bottle, adjust the power of the microwave reactor to 500W, start the microwave reactor and raise the temperature from room temperature to 230°C at a heating rate of 45°C / min, then react at a constant temperature for 20 minutes, turn off the microwave reactor, stop the reaction, cool naturally to room temperature, and put the reaction The mixture in the bottle was washed with distilled water, dried, recrystallized from ethanol, and weighed to obtain 2.42 g of (IIa2) as a yellow solid with a yield of 93.0%. mp:179~181℃.IRν(cm -1 ):3173,2919,1755,1628,1431,1331,1255,1120,1008,920,803,750,703,614; 1 H-NMR (400MHz, DMSO-d 6 ):9.27(s,1H),8.69(d,J=5.0Hz,1H),8.41(d,J=6.7Hz,1H),8.01(d,J=2.4Hz,1H),7.97(d,J =7.9Hz,1H),7.6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com